Calculate the cell potential (Ecell) of the following cell at 298 K . Ag(s) | AgNO3 (0.01 M) || AgNO3 (1.0 M) | Ag(s)
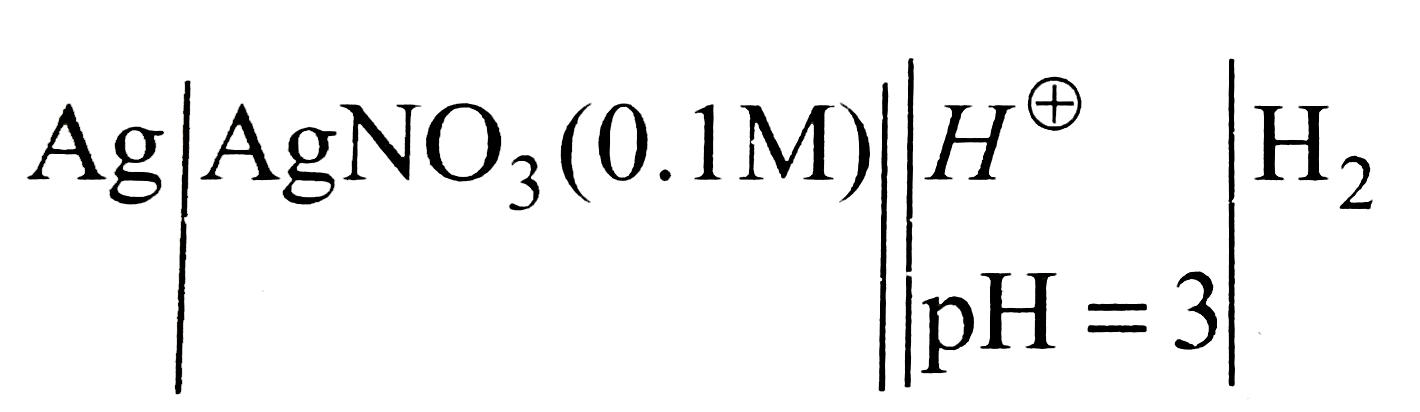
A silver electrode dipping in AgNO(3) solution (0.1M) is combined salt bridge with a hydrogen electrode dipping in a solution of pH=3(at 25^(@)C). If the standard reduction potential of the silver electrode
![What kind of reference electrode can be used in ionic liquids aqueous solution, for example, [BMIM][BF4] (aq)? | ResearchGate What kind of reference electrode can be used in ionic liquids aqueous solution, for example, [BMIM][BF4] (aq)? | ResearchGate](https://www.researchgate.net/profile/George-Chen-5/post/What_kind_of_reference_electrode_can_be_used_in_ionic_liquids_aqueous_solution_for_example_BMIMBF4_aq/attachment/59d6261f79197b8077984699/AS%3A320635132153856%401453456780434/download/Reference+electrode+with+salt+bridge.jpg)
What kind of reference electrode can be used in ionic liquids aqueous solution, for example, [BMIM][BF4] (aq)? | ResearchGate
25. Two concentration cells of Ag with Ag electrode in AgNO3.In first cell,concentration of one electrode is `1M and other electrode is .1M and emf is .06V.In second cell concentration of one