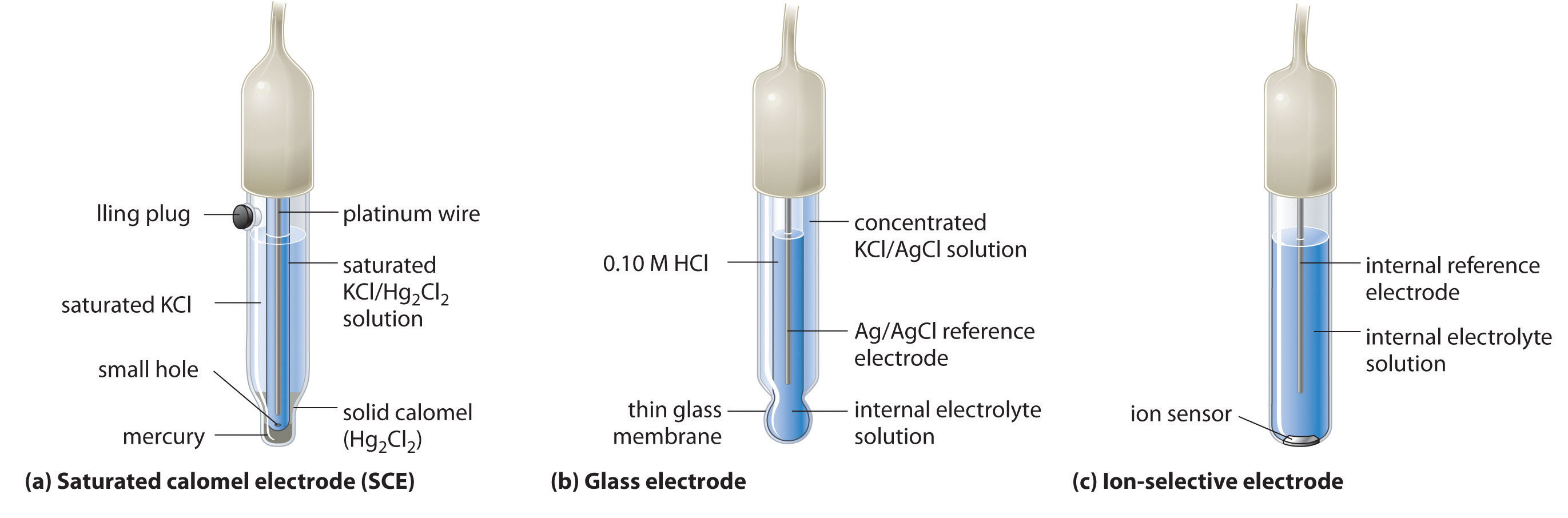
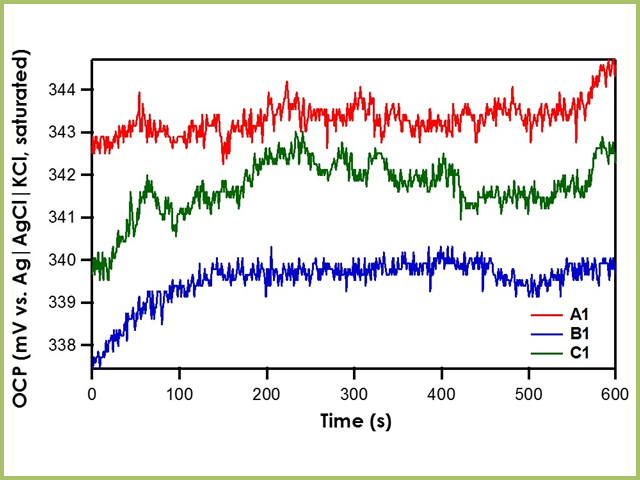
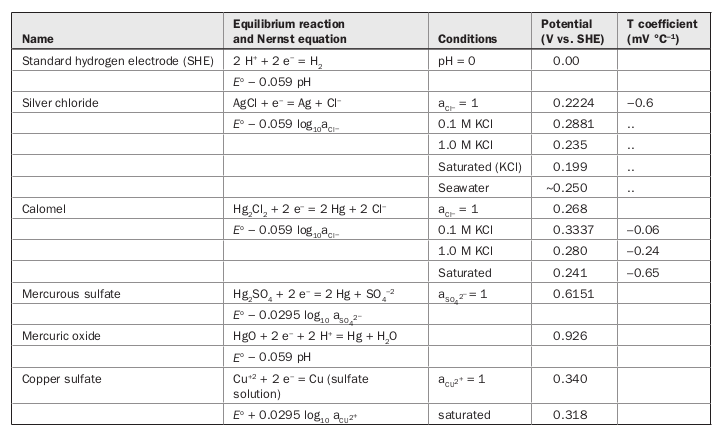
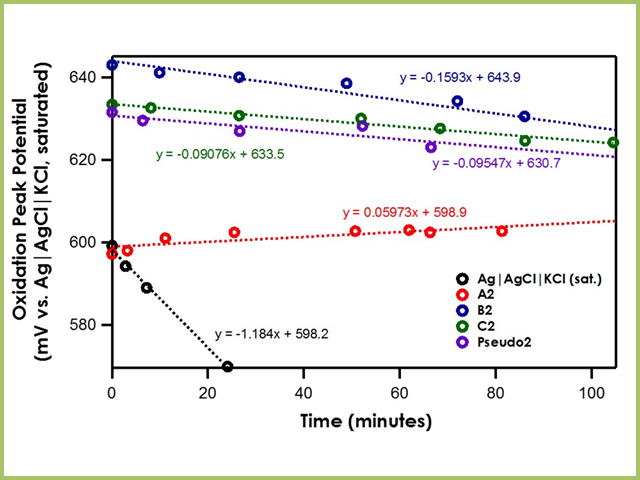
Figure S2. Potential calibration of the Ag/AgCl reference electrode in... | Download Scientific Diagram

Leakless, Bipolar Reference Electrodes: Fabrication, Performance, and Miniaturization | Analytical Chemistry

Silver Ion Electrode / Silver Ion Reference Electrode / Silver Nitrate Reference Electrode|Power Tool Accessories| - AliExpress
25. Two concentration cells of Ag with Ag electrode in AgNO3.In first cell,concentration of one electrode is `1M and other electrode is .1M and emf is .06V.In second cell concentration of one
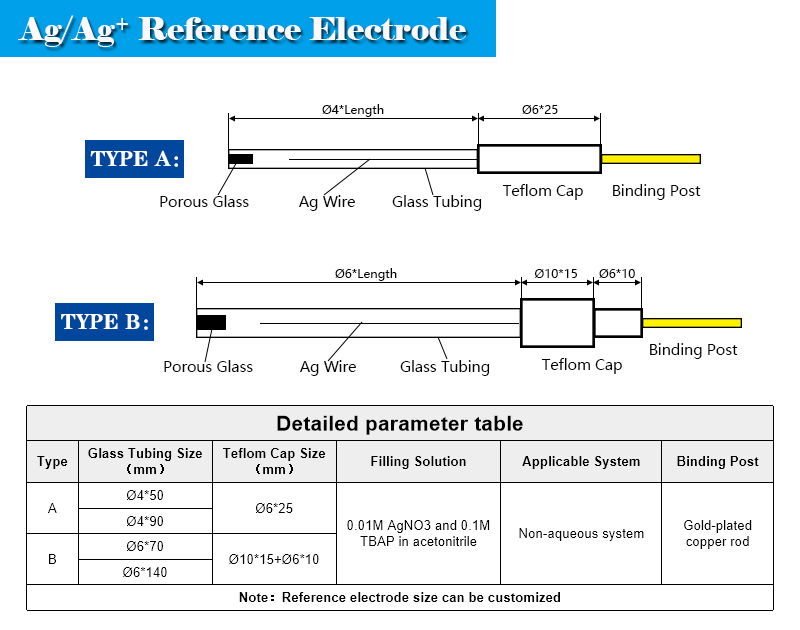
Non-aqueous Silver/Silver Ion Reference Electrode Ag/Ag+ φ4*50mm Glass Rod - Electrodes - Dekresearch

Calculate the cell potential (Ecell) of the following cell at 298 K . Ag(s) | AgNO3 (0.01 M) || AgNO3 (1.0 M) | Ag(s)
![SOLVED: 4.Calculate the potential of the cell consisting of a silver electrode dipping in a silver nitrate solution with [Agt] =0.0100 M and a SCE reference electrode. E' =+0.799V for Ag + SOLVED: 4.Calculate the potential of the cell consisting of a silver electrode dipping in a silver nitrate solution with [Agt] =0.0100 M and a SCE reference electrode. E' =+0.799V for Ag +](https://cdn.numerade.com/ask_images/6e8c2a727dbb4feba00a92f5c1749456.jpg)
SOLVED: 4.Calculate the potential of the cell consisting of a silver electrode dipping in a silver nitrate solution with [Agt] =0.0100 M and a SCE reference electrode. E' =+0.799V for Ag +
![What will be the reduction potential for the following half - cell reaction at 298 K?(Given: [Ag^ + ] = 0.1 M and E^ocell = + 0.80 V) What will be the reduction potential for the following half - cell reaction at 298 K?(Given: [Ag^ + ] = 0.1 M and E^ocell = + 0.80 V)](https://dwes9vv9u0550.cloudfront.net/images/5359922/bac1059b-faa8-4a13-917f-19241571bf3d.jpg)
What will be the reduction potential for the following half - cell reaction at 298 K?(Given: [Ag^ + ] = 0.1 M and E^ocell = + 0.80 V)
![CV for the reduction of 0.8 mM AgNO3 in [C4mpyrr][NTf2] on a platinum... | Download Scientific Diagram CV for the reduction of 0.8 mM AgNO3 in [C4mpyrr][NTf2] on a platinum... | Download Scientific Diagram](https://www.researchgate.net/publication/210186463/figure/fig5/AS:669326013104160@1536591165749/CV-for-the-reduction-of-08-mM-AgNO3-in-C4mpyrrNTf2-on-a-platinum-microelectrode.png)