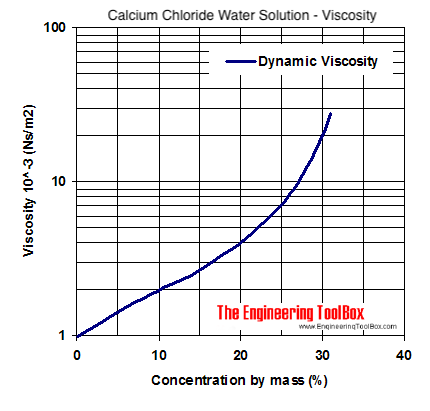
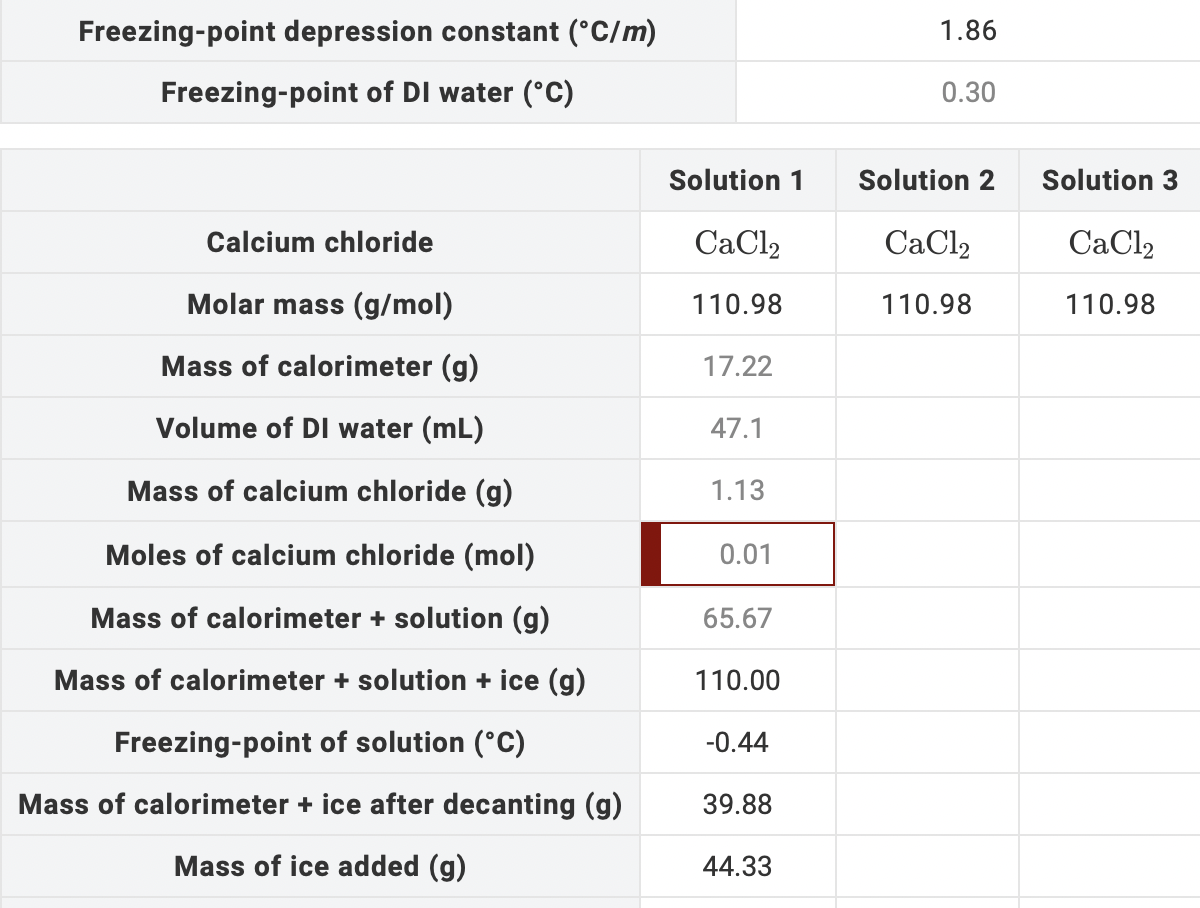
If Kf = 1.86 C/m, how many grams of calcium chloride must be added to 1.0 L of water (assume a density of 1.0 g/mL) to lower the freezing point 4.0 degrees

罗渽民 on Twitter: "“Calcium chloride forms brine rapidly, which lowers the freezing point of water and melts snow and ice quickly.” JAEMIN very knowledgeable. He doesn't just speak about it. He really
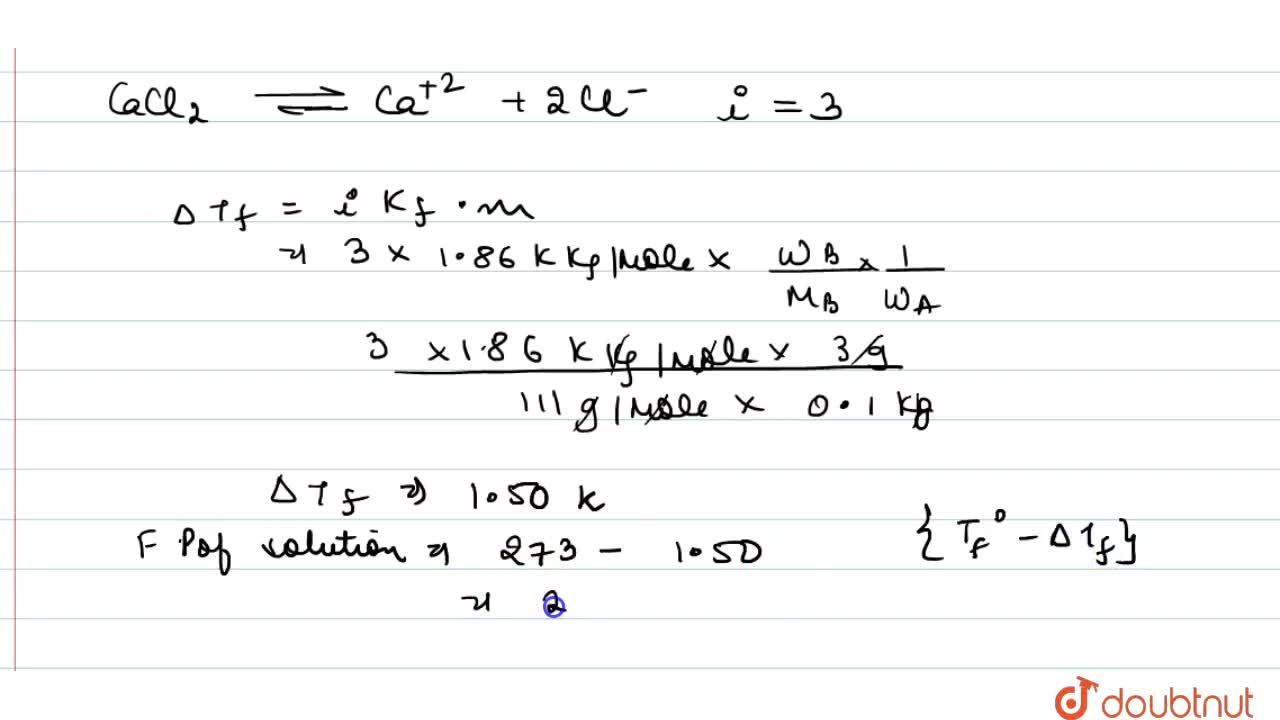
Calculate the freezing point of a solution when 3 g of CaCI(2) (M=111 g mol^(-1)) was dissolved in 100g of water assuming that CaCI(2) undergoes complete ionisation (K(f) "for water"=1.86 K kg

Calculate the freezing point of a solution when 3 g of CaCl2(M = 111 g mol^-1) was dissolved in 100 g of water.assuming CaCl2 Undergoes complete ionisation (Kf for water = 1.86 K kg mol^-1)
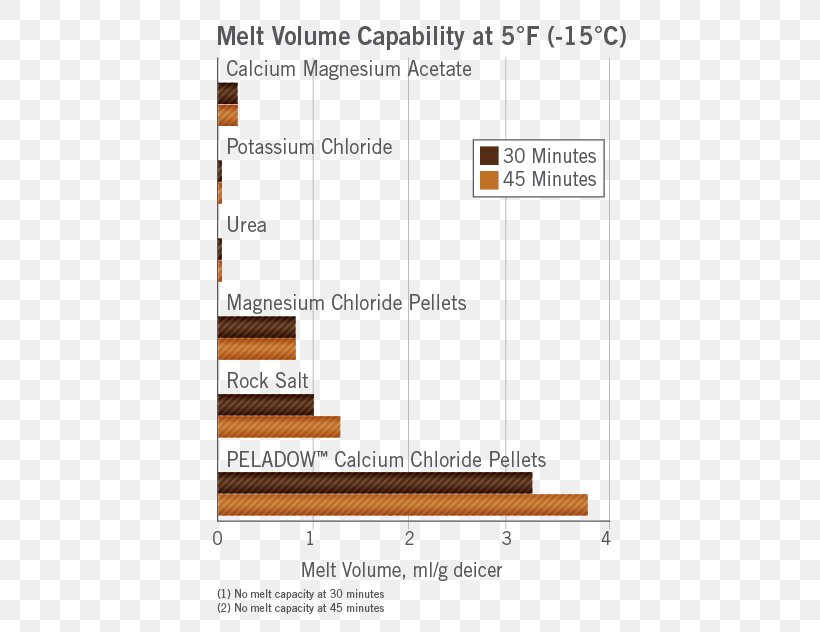
Sodium Chloride Melting Calcium Chloride Freezing-point Depression Deicing, PNG, 459x632px, Sodium Chloride, Area, Brine, Calcium,

Energies | Free Full-Text | An Aqueous CaCl2 Solution in the Condenser/Evaporator Instead of Pure Water: Application for the New Adsorptive Cycle “Heat from Cold”
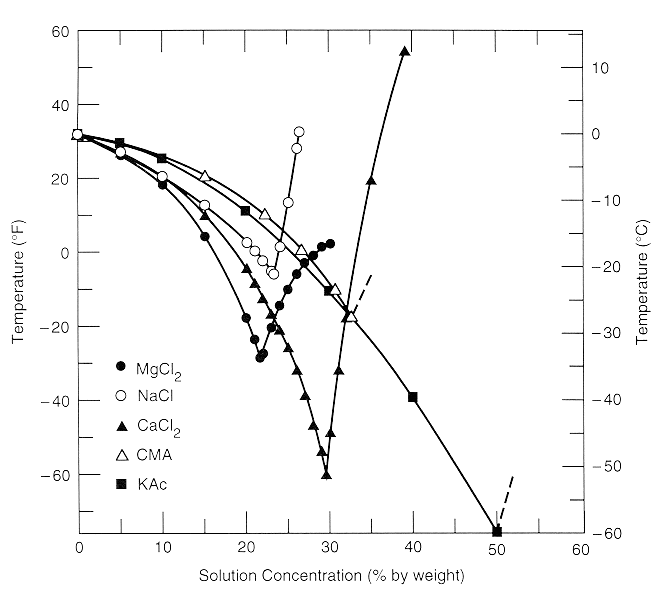
Recommended Anti-Icing Practices - Manual of Practice for An Effective Anti-Icing Program , June 1996 - FHWA-RD-95-202

The influence of calcium chloride deicing salt on phase changes and damage development in cementitious materials - ScienceDirect
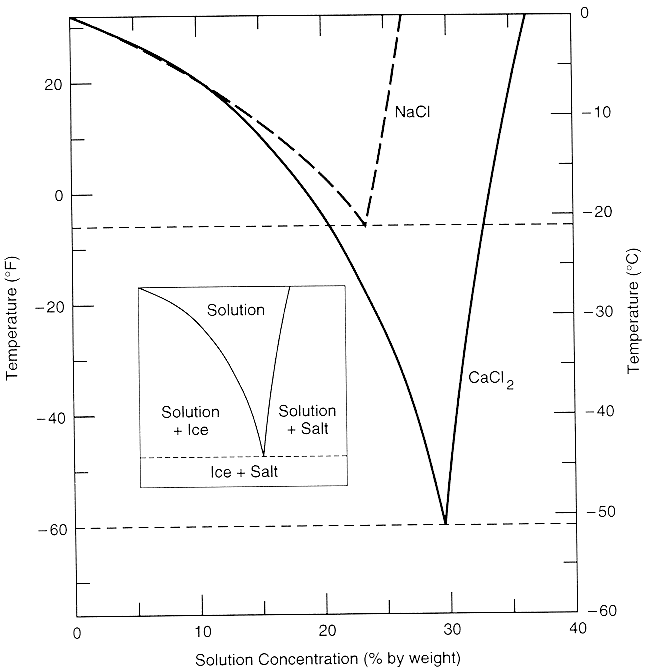


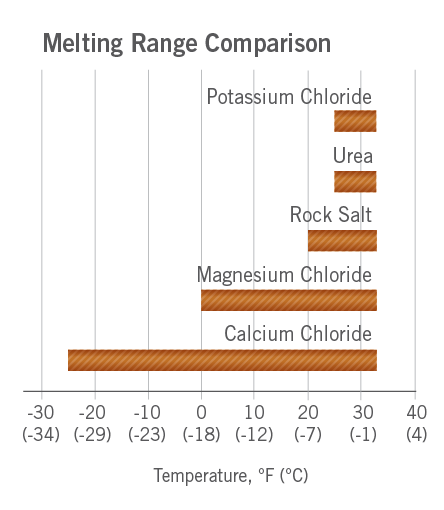




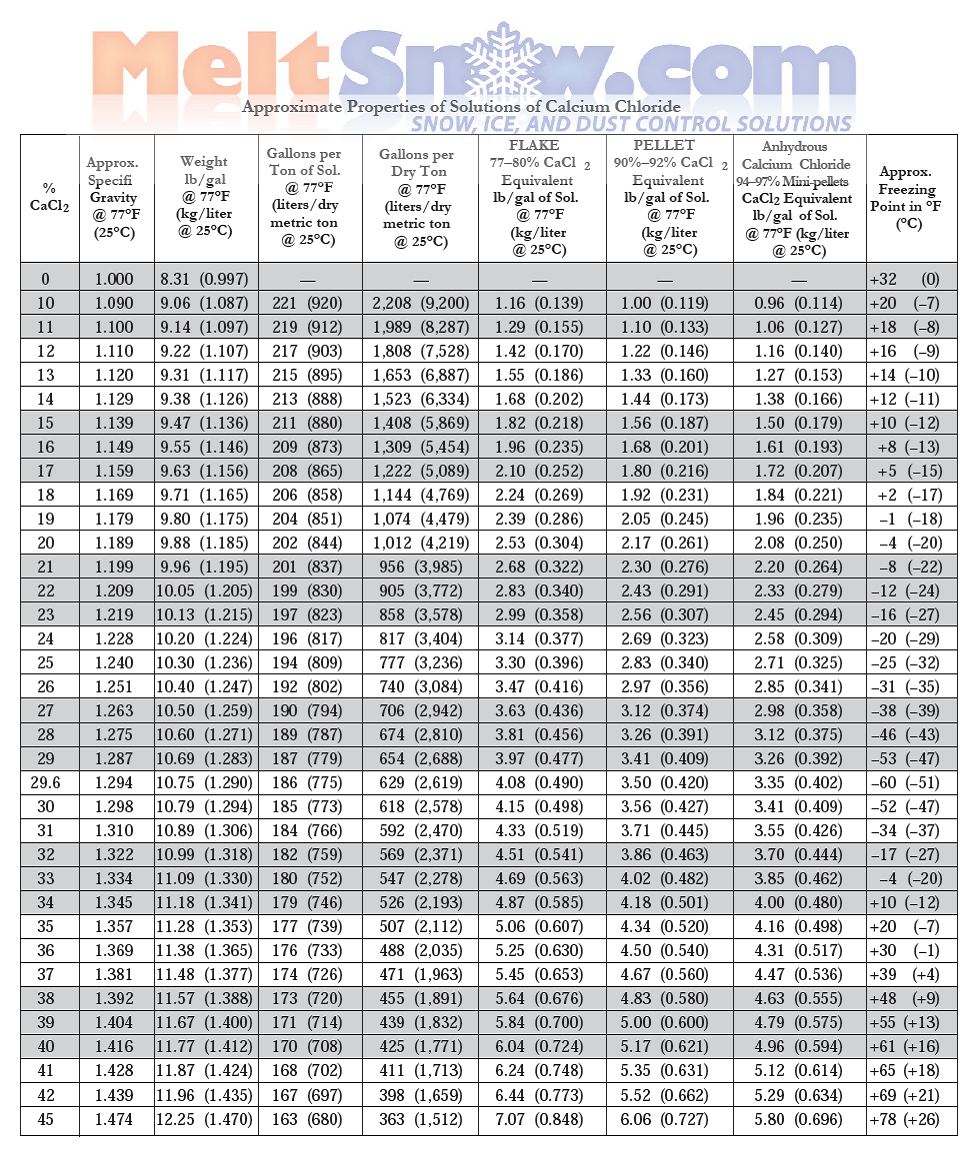

![Phase diagram of calcium chloride and water compound [8] | Download Scientific Diagram Phase diagram of calcium chloride and water compound [8] | Download Scientific Diagram](https://www.researchgate.net/publication/282899613/figure/fig1/AS:297416440991773@1447921013811/Phase-diagram-of-calcium-chloride-and-water-compound-8.png)