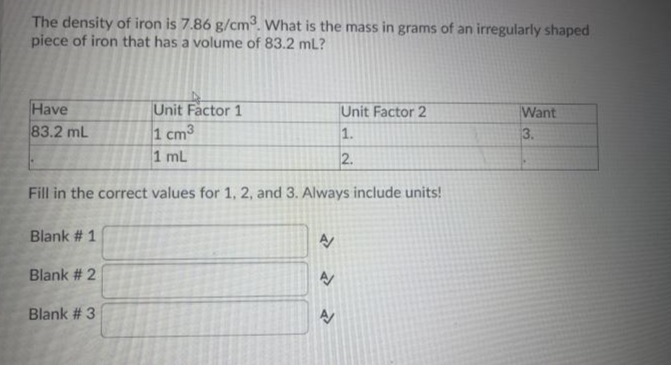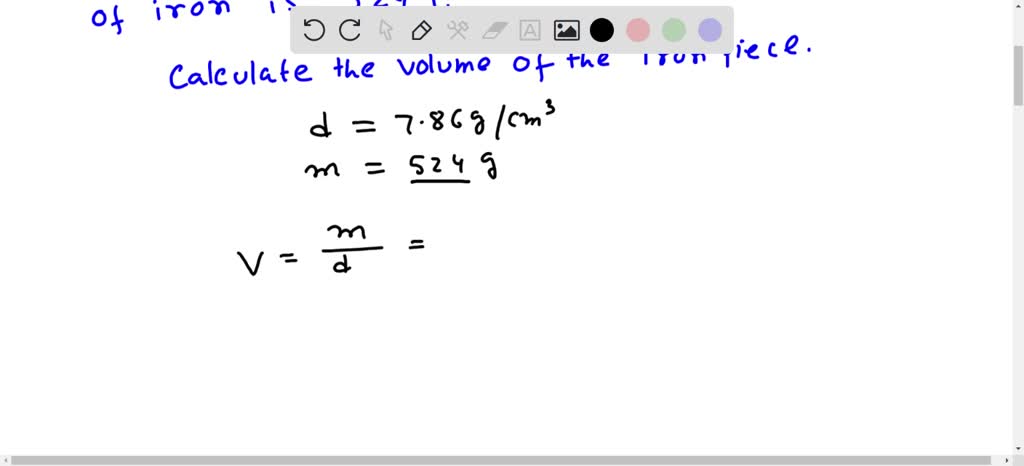
SOLVED: The density of iron is 7.86 g/cm3. What is the volume in milliliters (mL) of an irregularly shaped piece of iron that has a mass of 524 g?

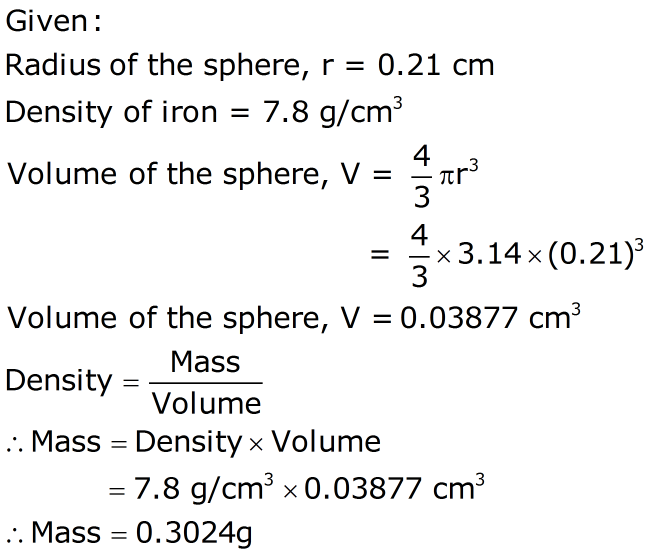
The density of iron is 7.87 g/cm^3 . If the atoms are spherical and closely packed. The mass of iron atom is 9.27 × 10^-26 kg. What is the volume of an iron atom?
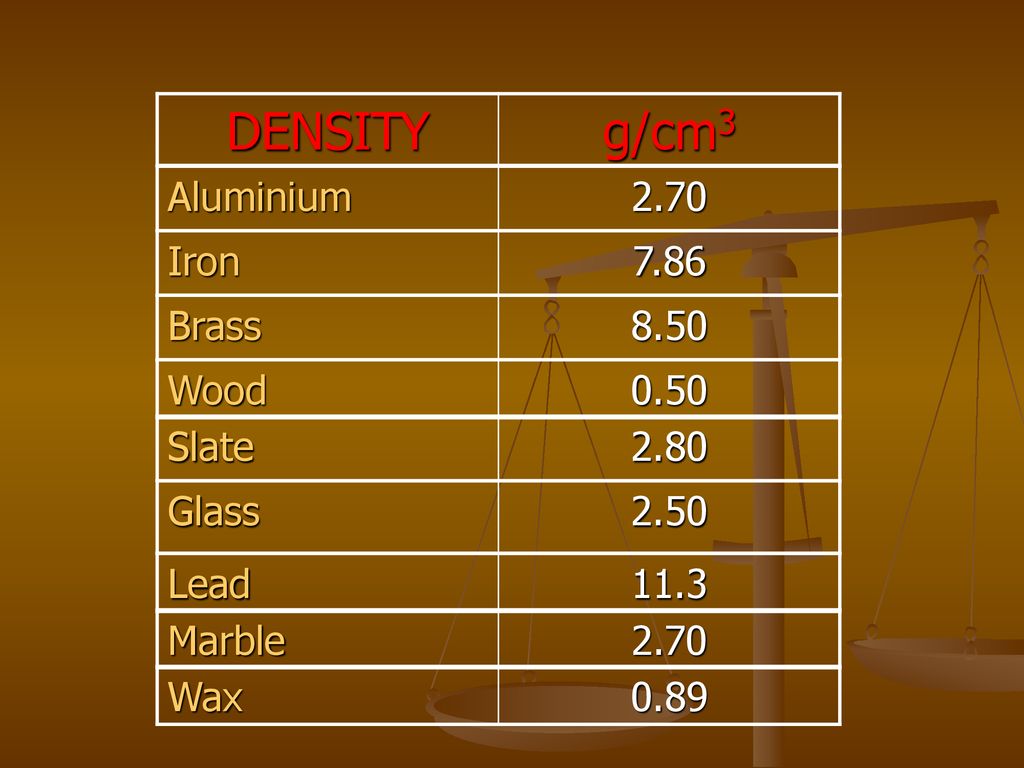
Table of Densities Material Density g/cm3 Water 1.0 Alcohol 0.8 Diamond 3.5 Iron 7.9 Gold 18.9 Lead 11.3 English oak 0.7 Ice 0.9

SOLVED: Iron has a density of 7.87 g/cm3. If 52.4 grams of iron is added to 75.0 mL of water in a graduated cylinder, to what volume reading will the water level
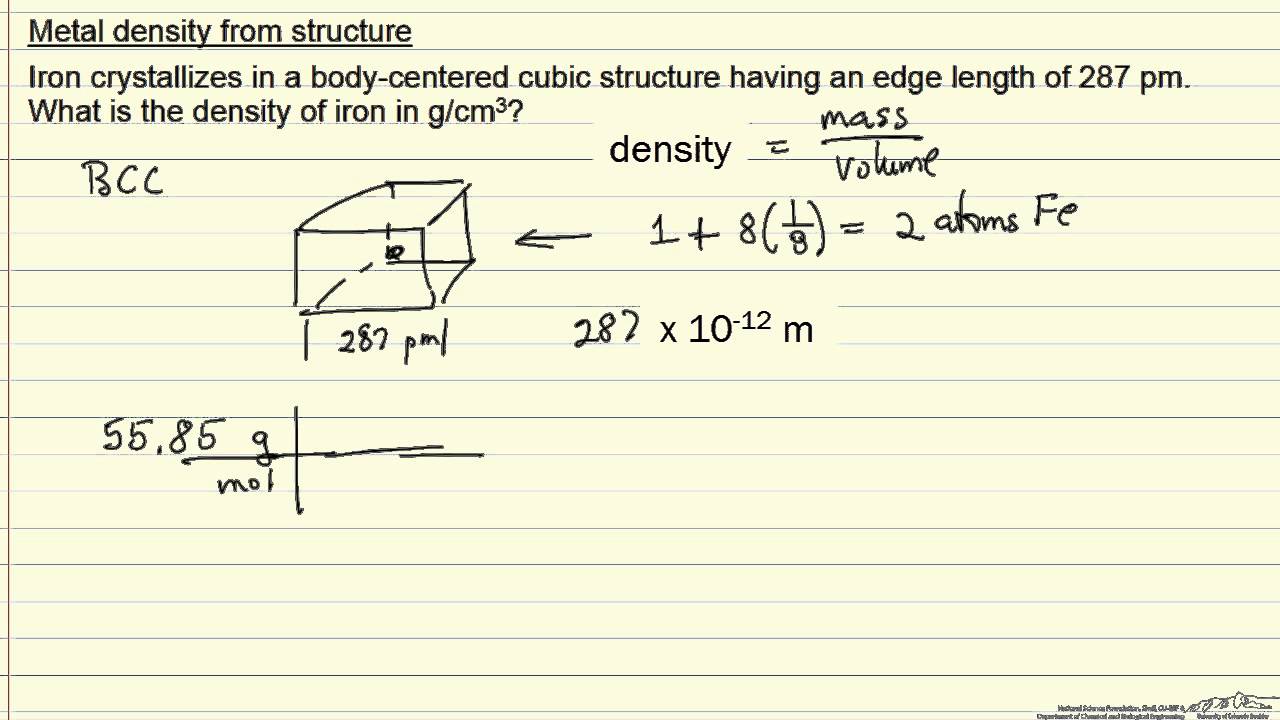
Iron has a body-centred cubic unit cell with a cell edge of 286.65 pm. The density of iron is 7.87g cm^–3. - Sarthaks eConnect | Largest Online Education Community
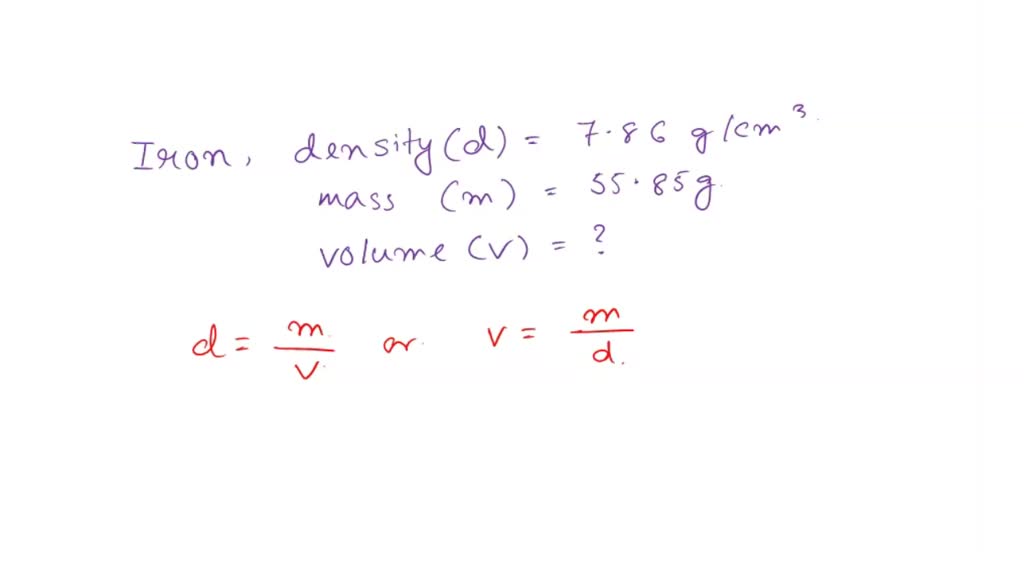
SOLVED: Iron has a density of 7.86 g/cm3. The volume occupied by 55.85 g of iron is Group of answer choices 2.8 cm3 439 cm3 0.141 cm3 7.11 cm3

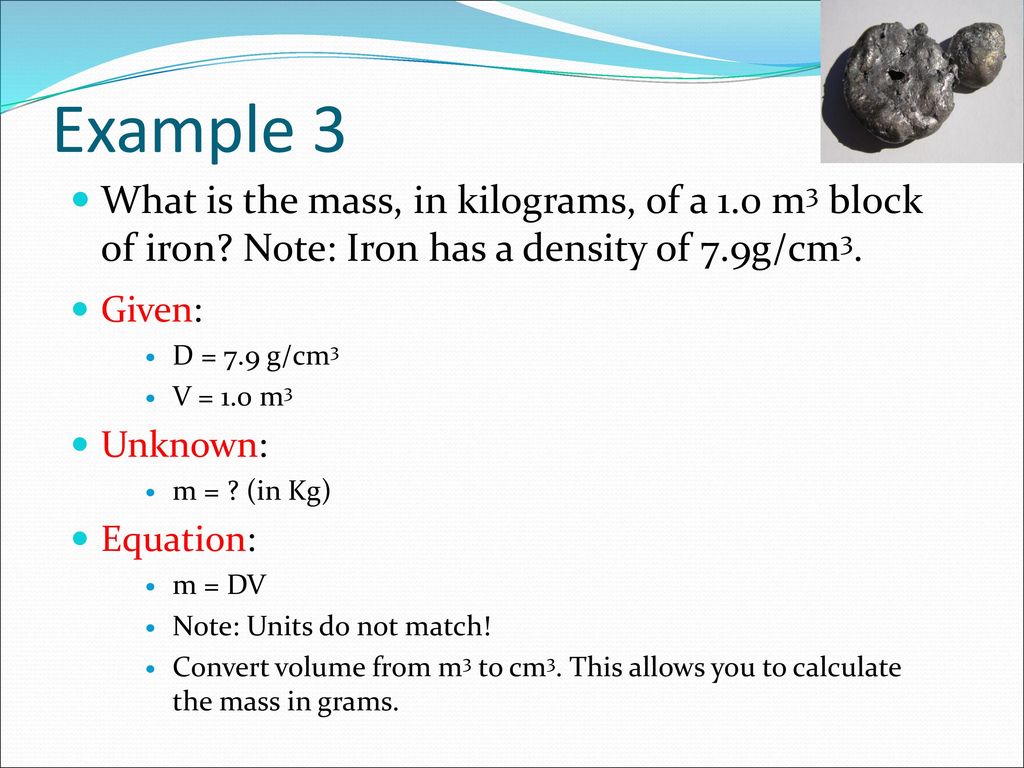
The density of iron is 7.87 g/mL. What is the mass of a 3.00 cm × 4.00 cm × 1.00 cm block of iron? - Brainly.in

Heat Treatment - What is steel density ? https://ift.tt/2U6roNw steel density The density of steel is in the range of 7.75 and 8.05 g/cm3 (7750 and 8050 kg/m3 or 0.280 and 0.291