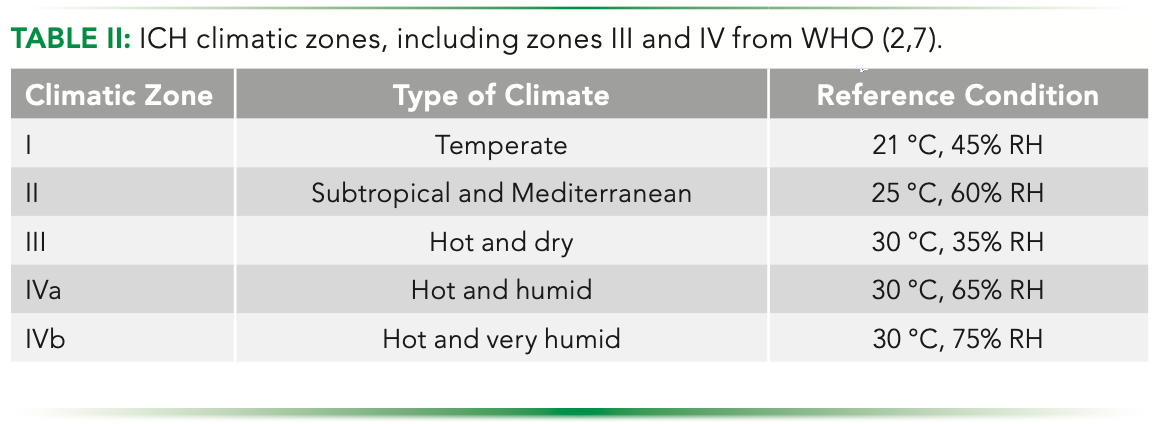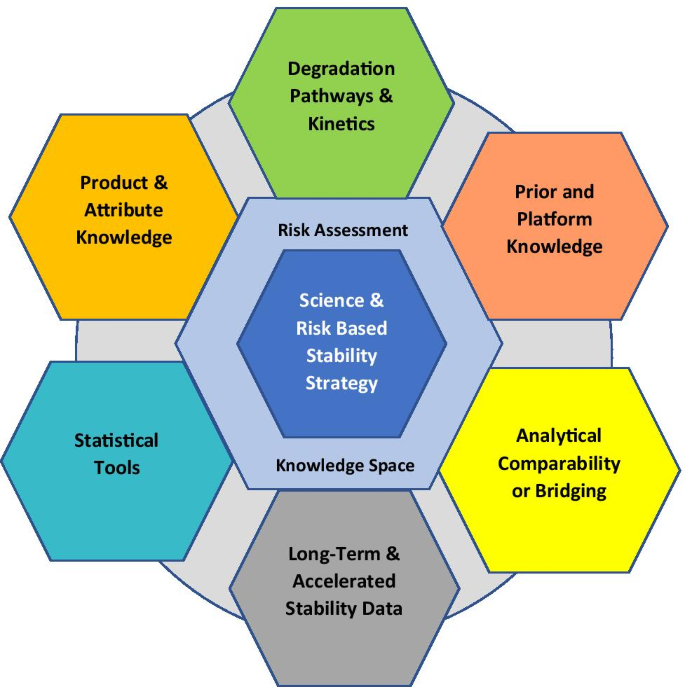
Considerations for Updates to ICH Q1 and Q5C Stability Guidelines: Embracing Current Technology and Risk Assessment Strategies | SpringerLink

Stability studies needed to define the handling and transport conditions of sensitive pharmaceutical or biotechnological products. - Abstract - Europe PMC

Figure 1 from Stability Studies Needed to Define the Handling and Transport Conditions of Sensitive Pharmaceutical or Biotechnological Products | Semantic Scholar

Stability Studies Needed to Define the Handling and Transport Conditions of Sensitive Pharmaceutical or Biotechnological Products | SpringerLink

Analytical, Bioanalytical, Stability-Indicating Methods: Key Part of Regulatory Submissions | IntechOpen
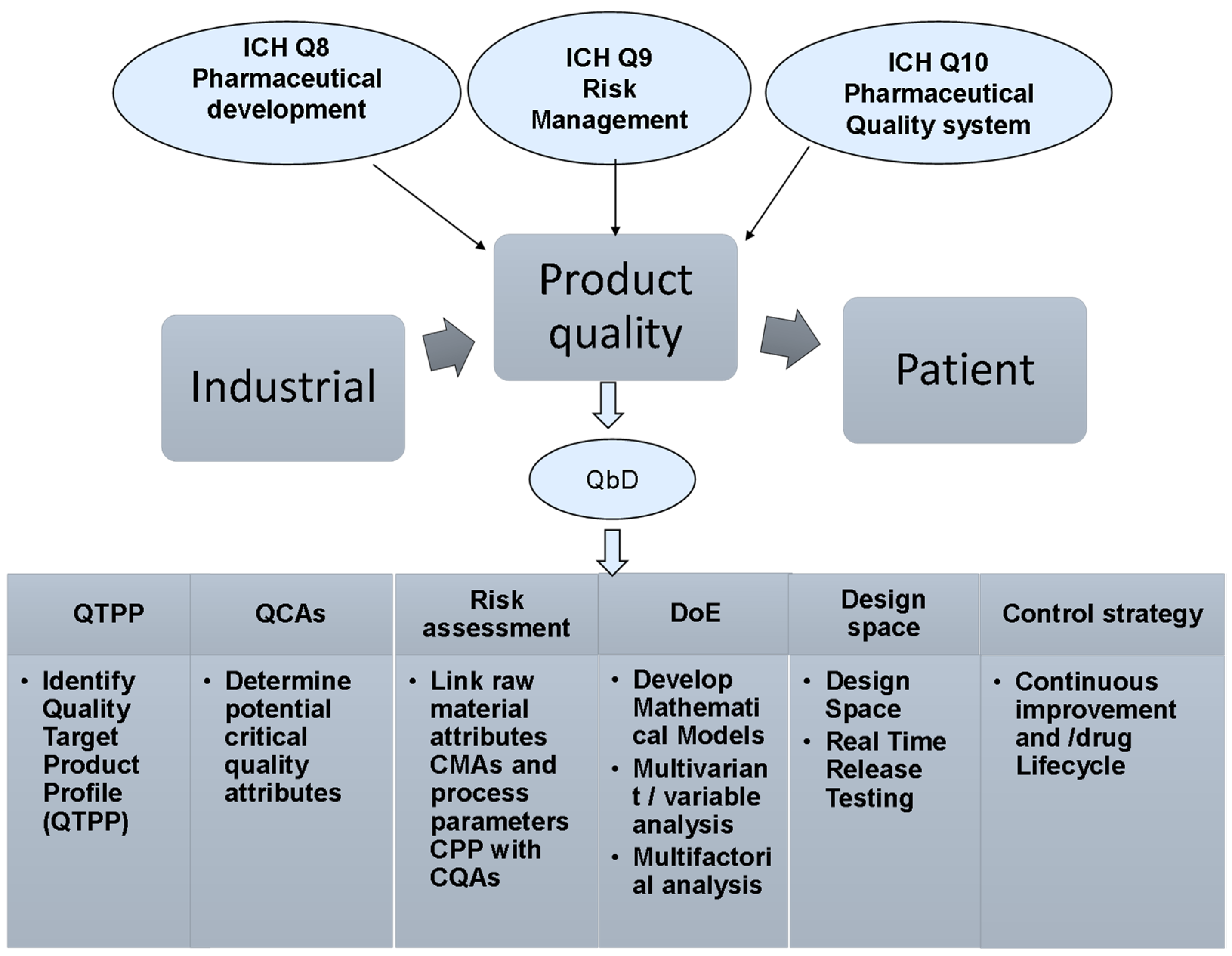
Molecules | Free Full-Text | Quality by Design Approach in Liposomal Formulations: Robust Product Development