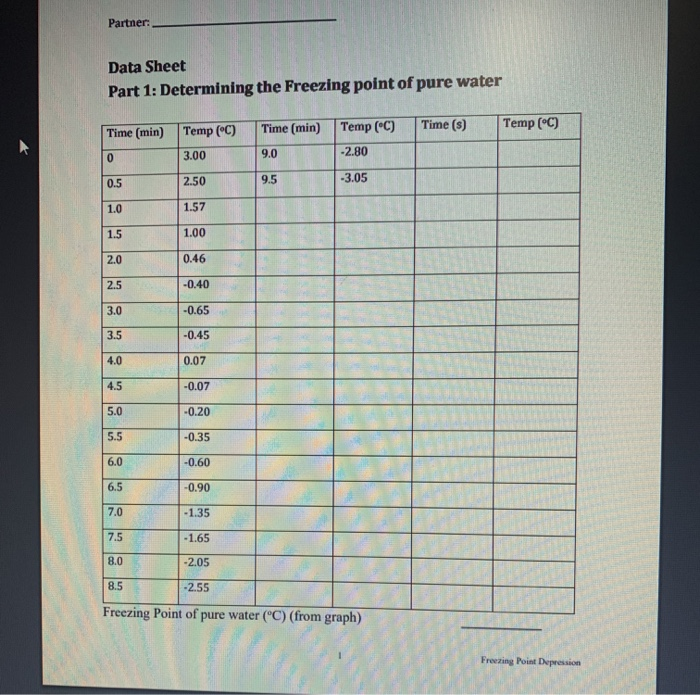
How do you find the freezing point of pure water from the freezing point depression equation? | Homework.Study.com

SOLVED: Must subtracted from the freezing point of pure water, which is The change In freezing point = predicted freezing point with - the actual freezing point: Compare O.0FC, in order to
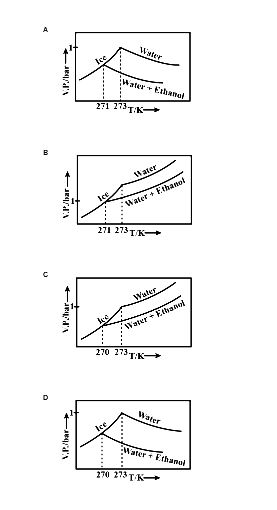
Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethanol to 500g of water changes the freezing point of the solution. Use the freezing point depression

Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1 ) in 250 g of water. ( Kf of water = 1.86 K kg mol^-1 ).

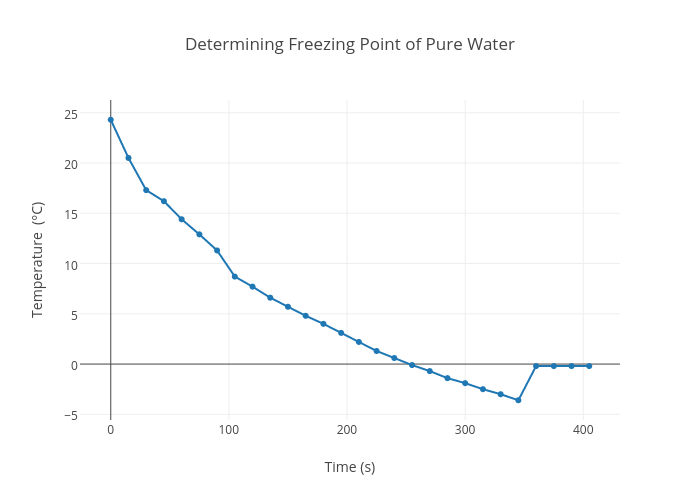
Comparison of pure water freezing using filtered MilliQ produced water... | Download Scientific Diagram

Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethanol to 500 g of water changes the freezing point of the solution. Use the freezing point

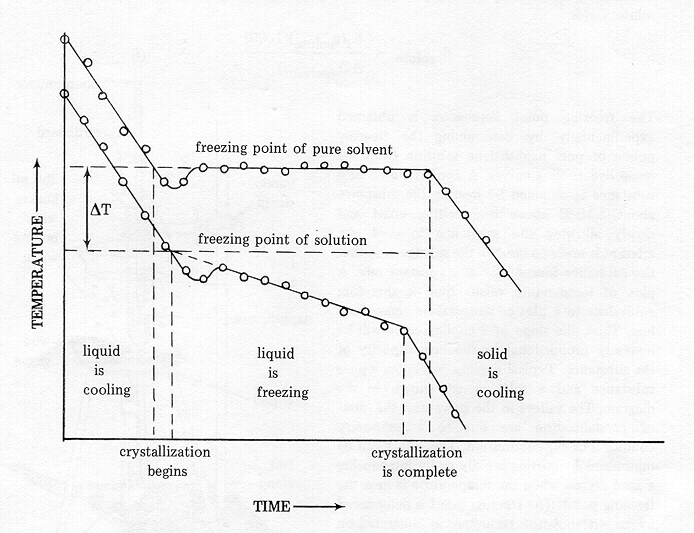
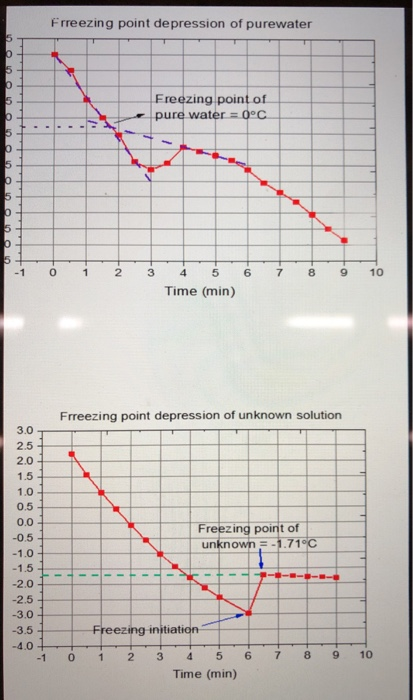
2 Typical freezing profile of frozen pure water and food systems. The... | Download Scientific Diagram
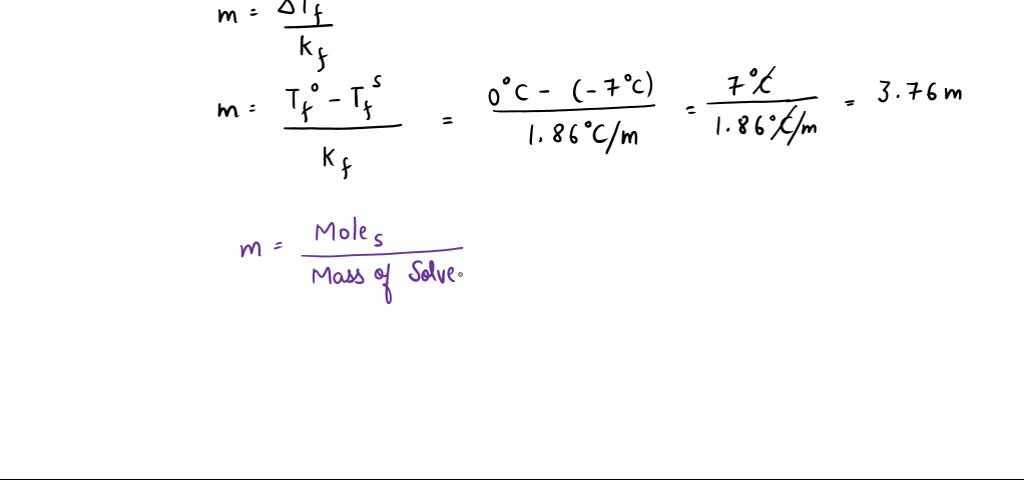
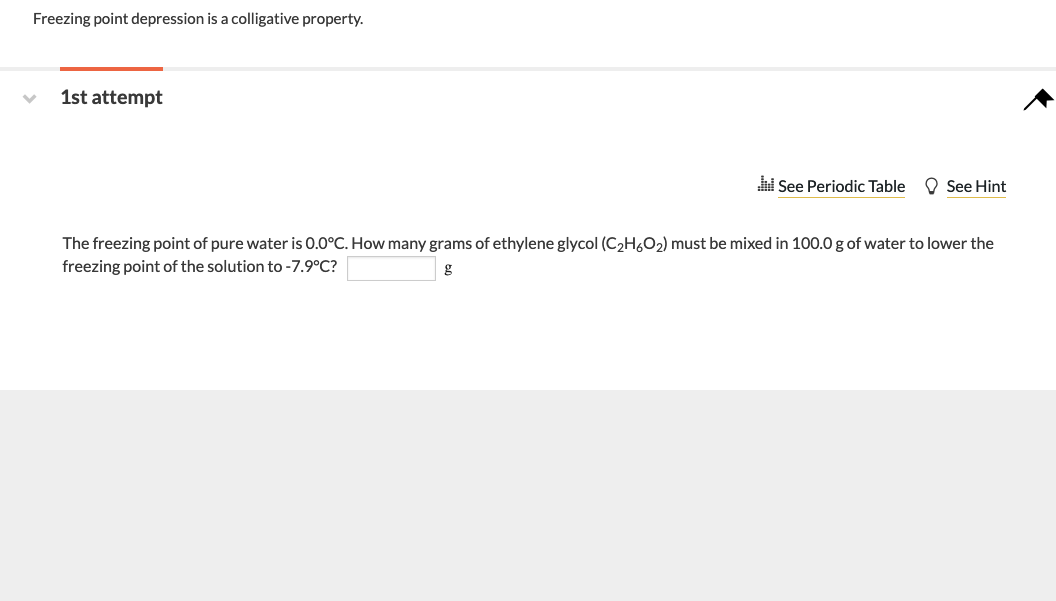
SOLVED: The freezing point of pure water is 0.0°C. How many grams of ethylene glycol (C2H6O2) must be mixed in 100.0 g of water to lower the freezing point of the solution

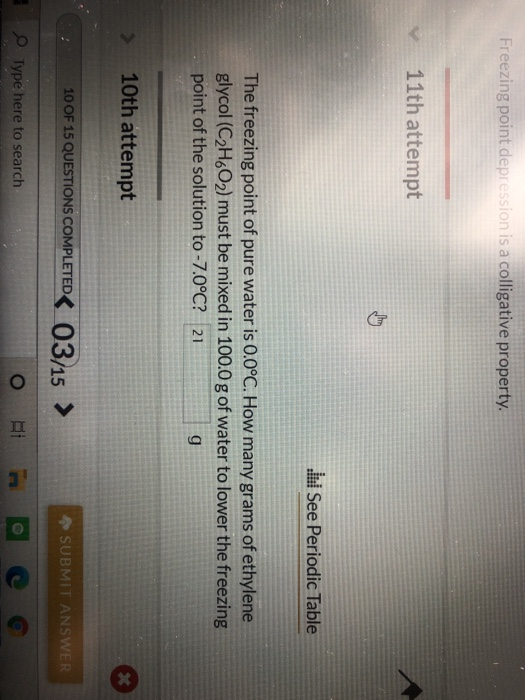
SOLVED: @ See page 526 15 Question (1 point) Freezing point depression Iligativc property 17th attempt See Periodic Table See Hint Feedback The freczing point of pure water is0.OFC. How many grams

A `5%` solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the free... - YouTube

A 5% (by mass) of cane sugar in water has freezing point of 271K . Calculate the freezing point of 5% glucose in water if freezing point of water is 273.15K .





:max_bytes(150000):strip_icc()/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)