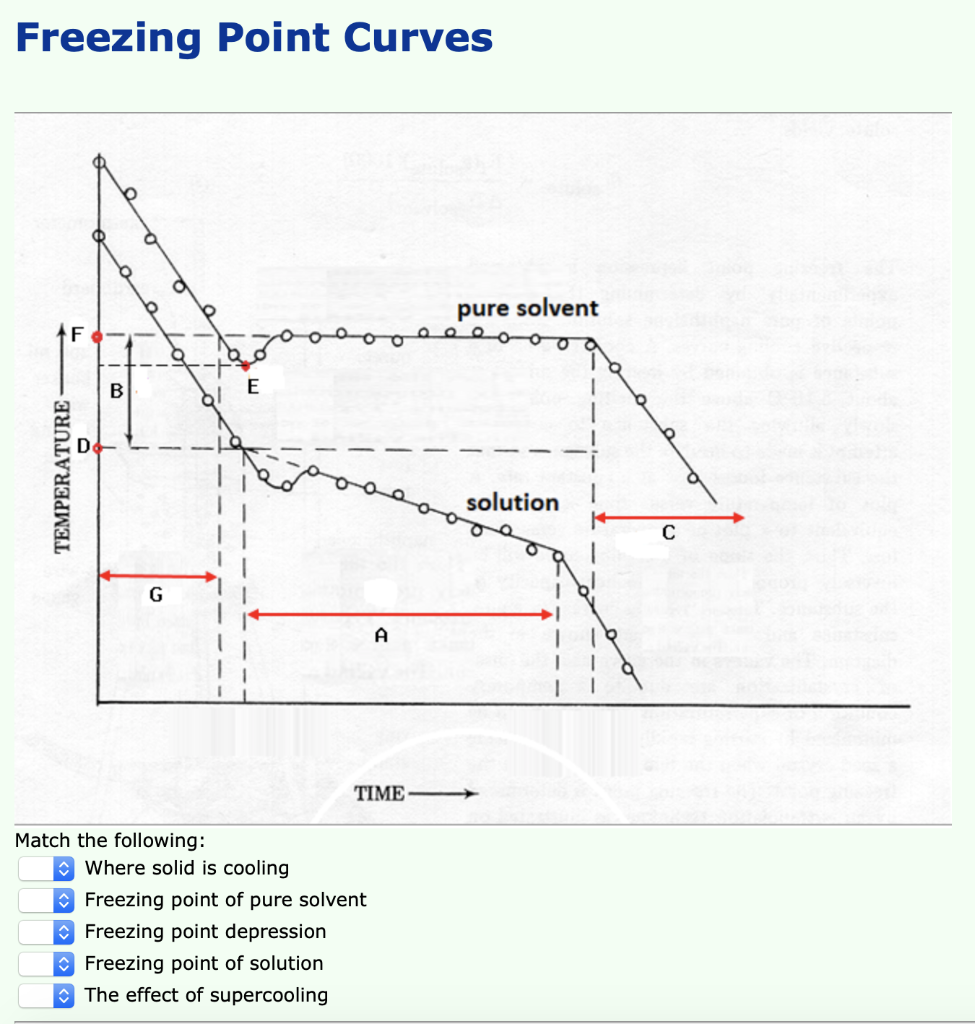
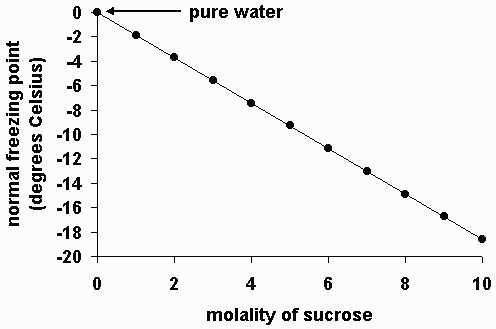
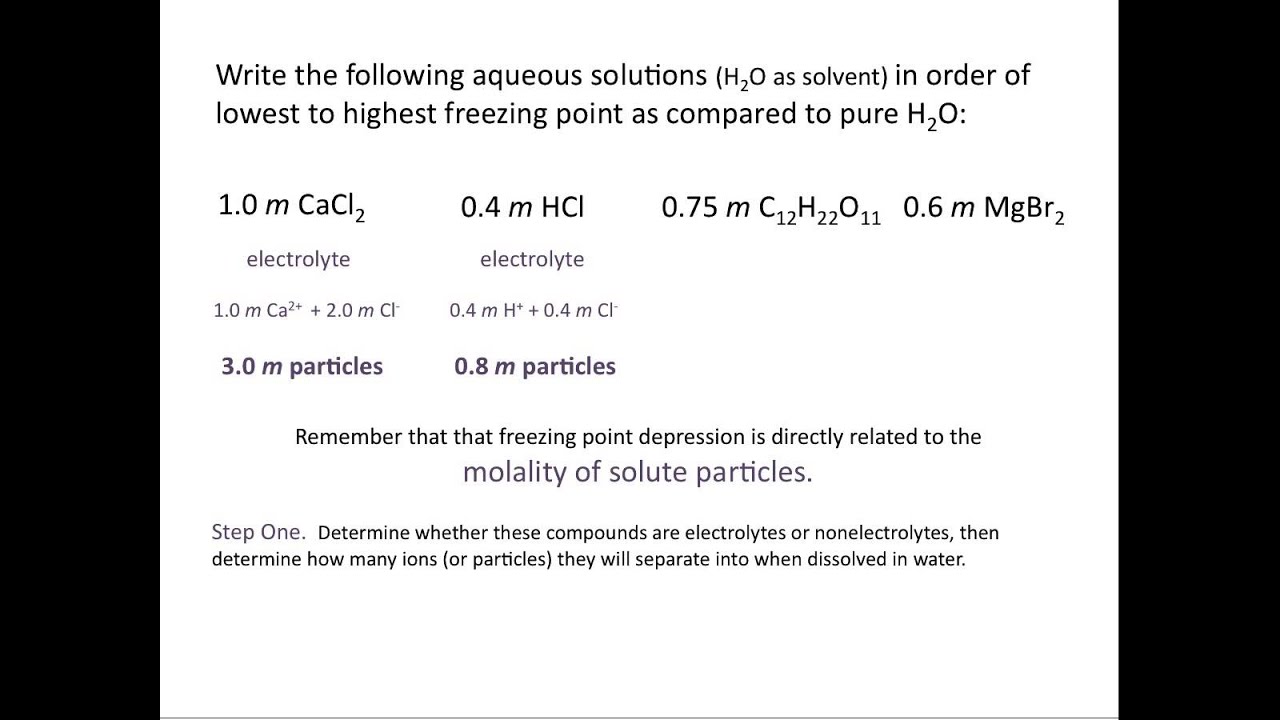
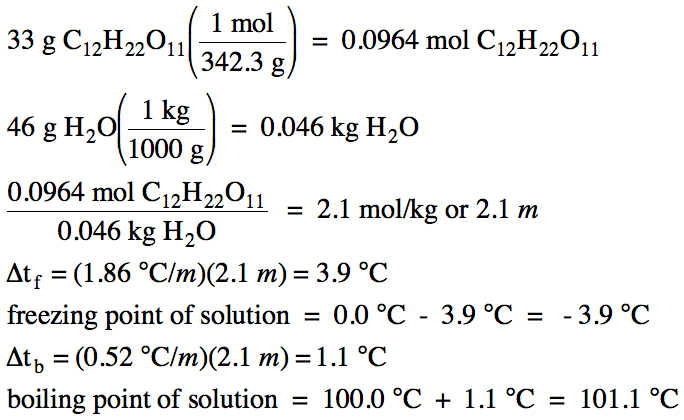Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1) in 250 g of water. - Sarthaks eConnect | Largest Online Education Community

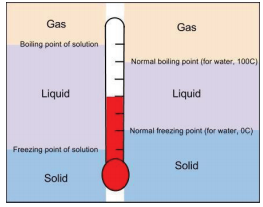
How to determine the freezing point of a solution, do you follow this process for every solution - Quora

SOLVED:Calculate the freezing point and melting point of a solution containing 7.55 g of ethylene glycol (C2 H6 O2) in 85.7 mL of ethanol. Ethanol has a density of 0.789 g / cm^3.

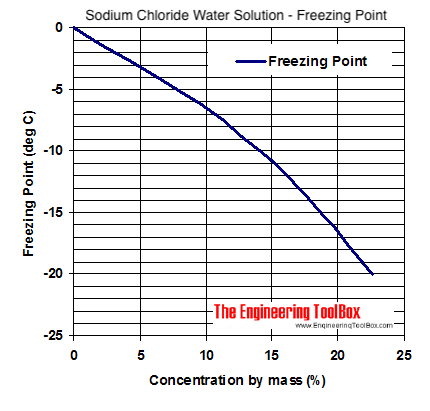
What is the freezing point of a solution containing 8.1g Br in 100g water assuming the acid to be 90%ionised(K(f)for water =1.8K "mole"^(-1))

Correct order of freezing point of following solutions(1)0 1m CaCl2(2)0 01m NaCl(3)0 01m K2SO4 - Chemistry - Solutions - 12724915 | Meritnation.com