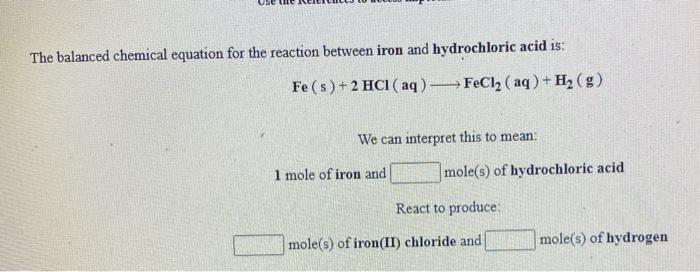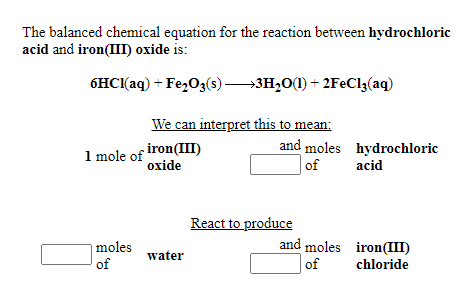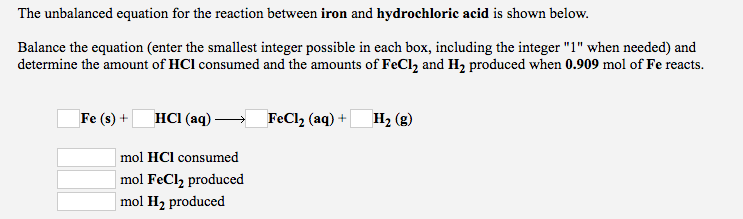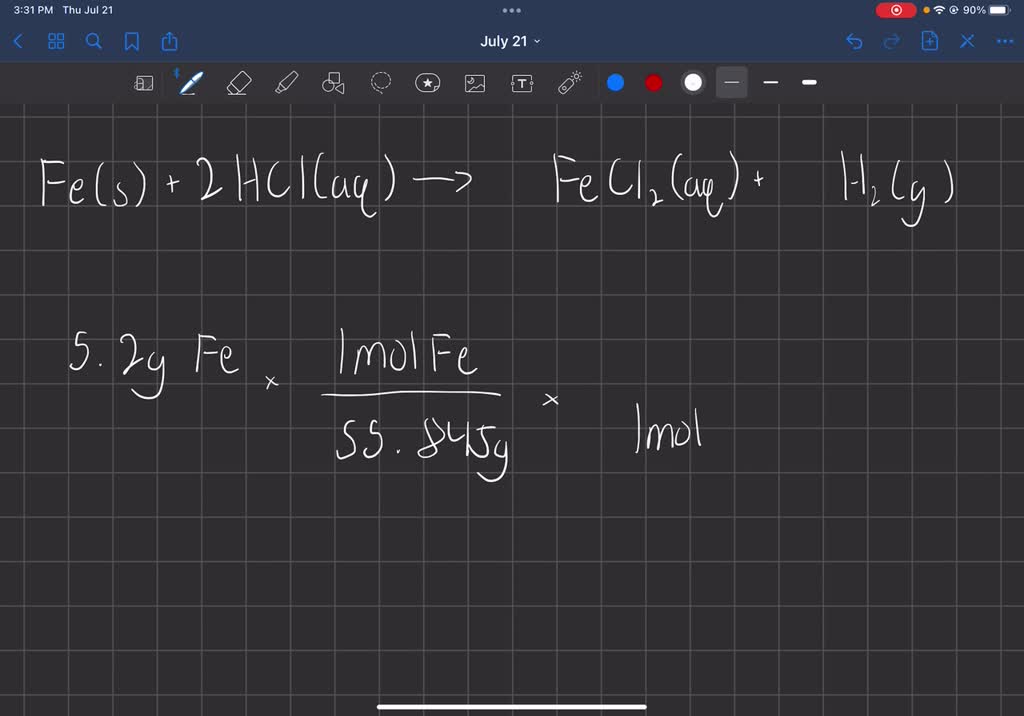
SOLVED: Iron reacts with hydrochloric acid to produce iron(II) chloride and hydrogen gas. Fe(s) + HCl(aq) → FeCl2(aq) + H2(g) What mass of H2(g) is produced from the reaction of 5.2 g

SOLVED: Solid iron (Il) sulfide reacts with hydrochloric acid to form hydrogen sulfide gas and aqueous iron (II) chloride: FeS (s) + 2 HCI (aq) –> H2S (g) + FeCl2 (aq) If

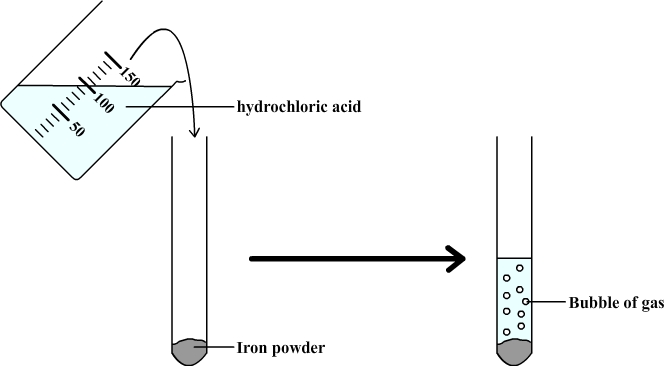
What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.... - YouTube

Laboratory apparatus to produce hydrogen sulphide from dilute hydrochloric acid and iron sulphide Stock Photo - Alamy

organic chemistry - Preference for tin or iron in the reduction of nitrobenzene - Chemistry Stack Exchange

Iron reacting with hydrochloric acid. Image 1 of 4. Iron (Fe) reacts with hydrochloric acid (HCl) to form iron (II) chloride (FeCl2). Bubbles of hydro Stock Photo - Alamy
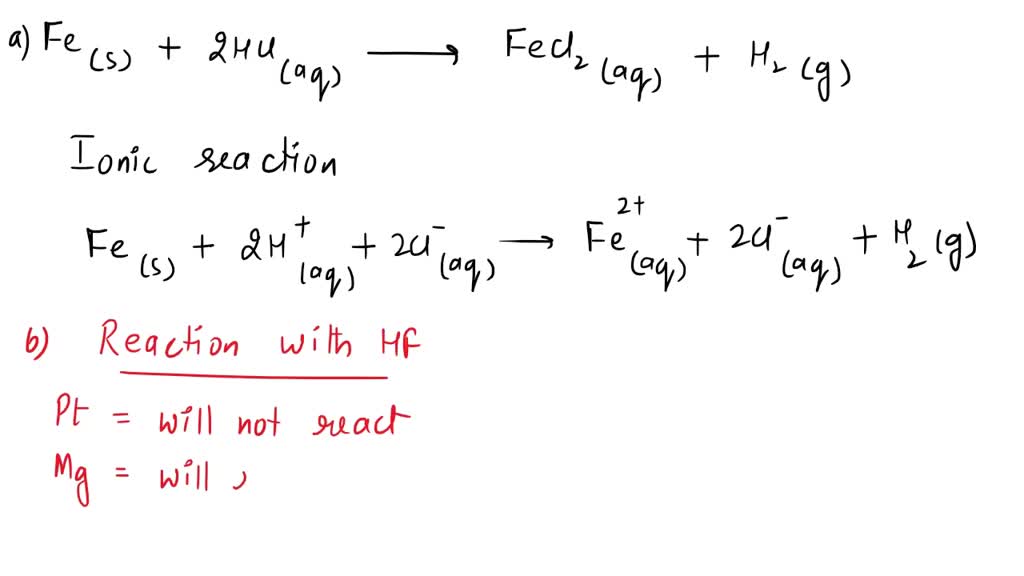
SOLVED: Write the net ionic equation for the reaction of iron with hydrochloric acid. Include phases. net ionic equation: Hydrofluoric acid reacts with metals in a similar fashion to hydrochloric acid. Predict

What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.a)Iron salt and water are produced.b)Chlorine gas and iron hydroxide are produced.c)No reaction takes place.d)Hydrogen gas and iron

Question Video: Recalling the Products of the Reaction between Iron Metal and Dilute Mineral Acids | Nagwa