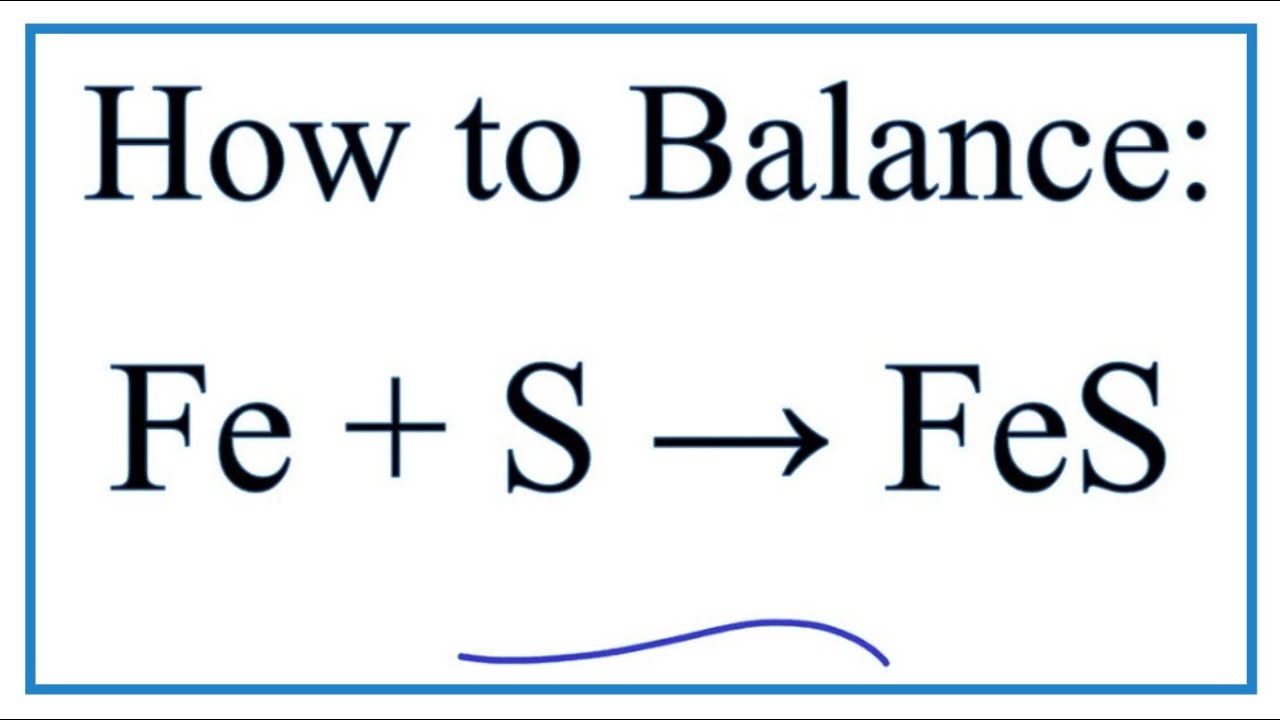
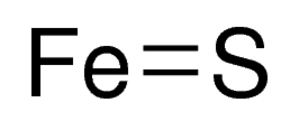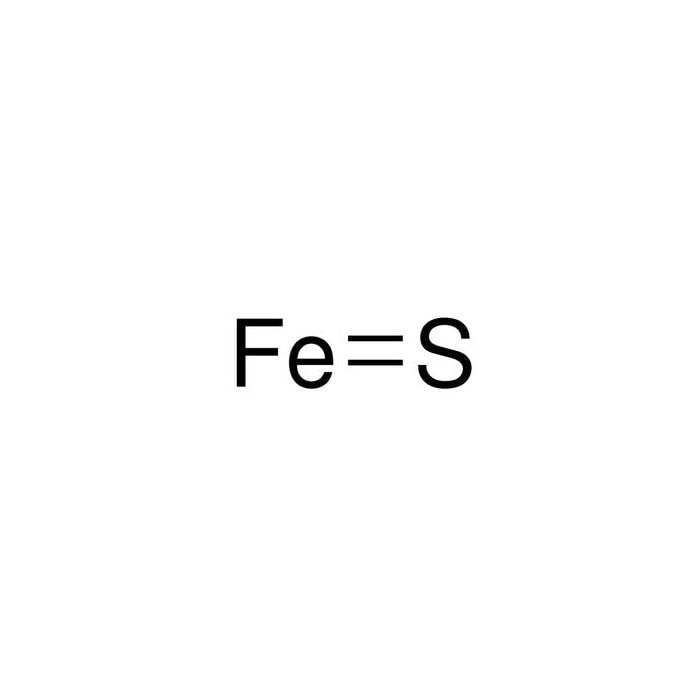SOLVED: For the compound iron (II) sulfide, answer the following questions: a. Write the correct chemical formula for iron (II) sulfide? b. How many atoms are in one formula unit of this
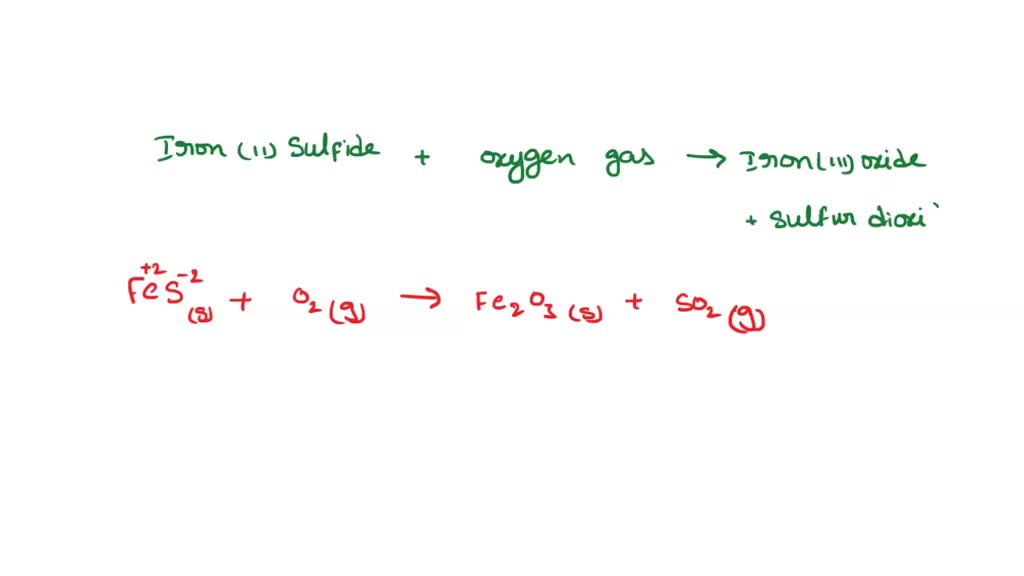
SOLVED: A student reacted iron (II) sulfide with oxygen gas to form iron ( III) oxide and sulfur dioxide. What is the balance equation? 4FeS(s) + 7020) 2Fe203(s) 4502() AFeS(s) + 402lg) 2Fe203(s)
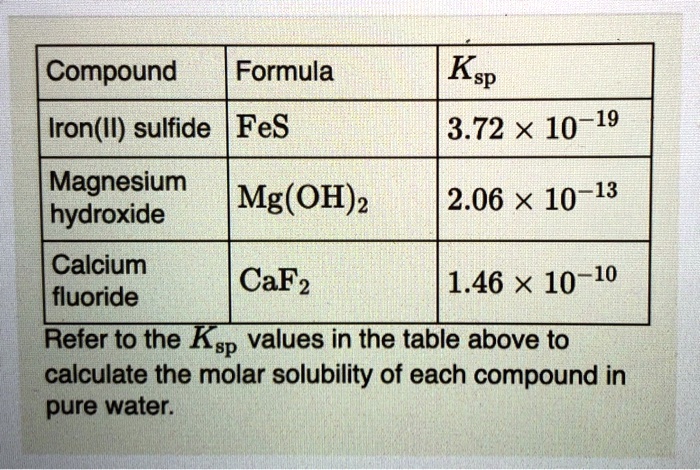
SOLVED: Compound Formula Ksp Iron(II) sulfide FeS 3.72 X 10-19 Magnesium Mg(OH)2 2.06 X 10-13 hydroxide Calcium CaF2 1.46 X 10-10 fluoride Refer to the Ksp values in the table above to