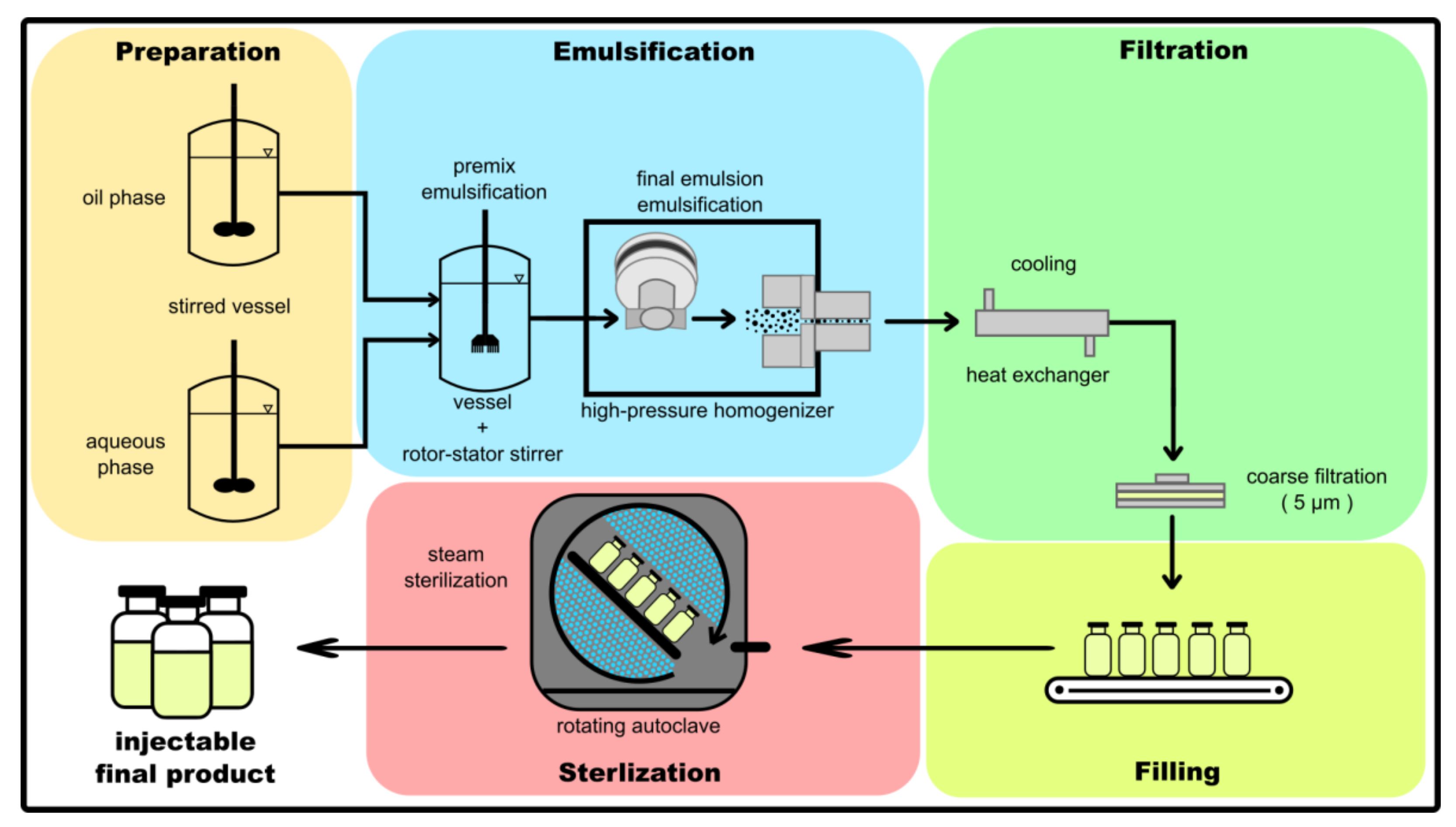
Facility Requirements For Biotech Plants | PDF | Verification And Validation | National Environmental Policy Act
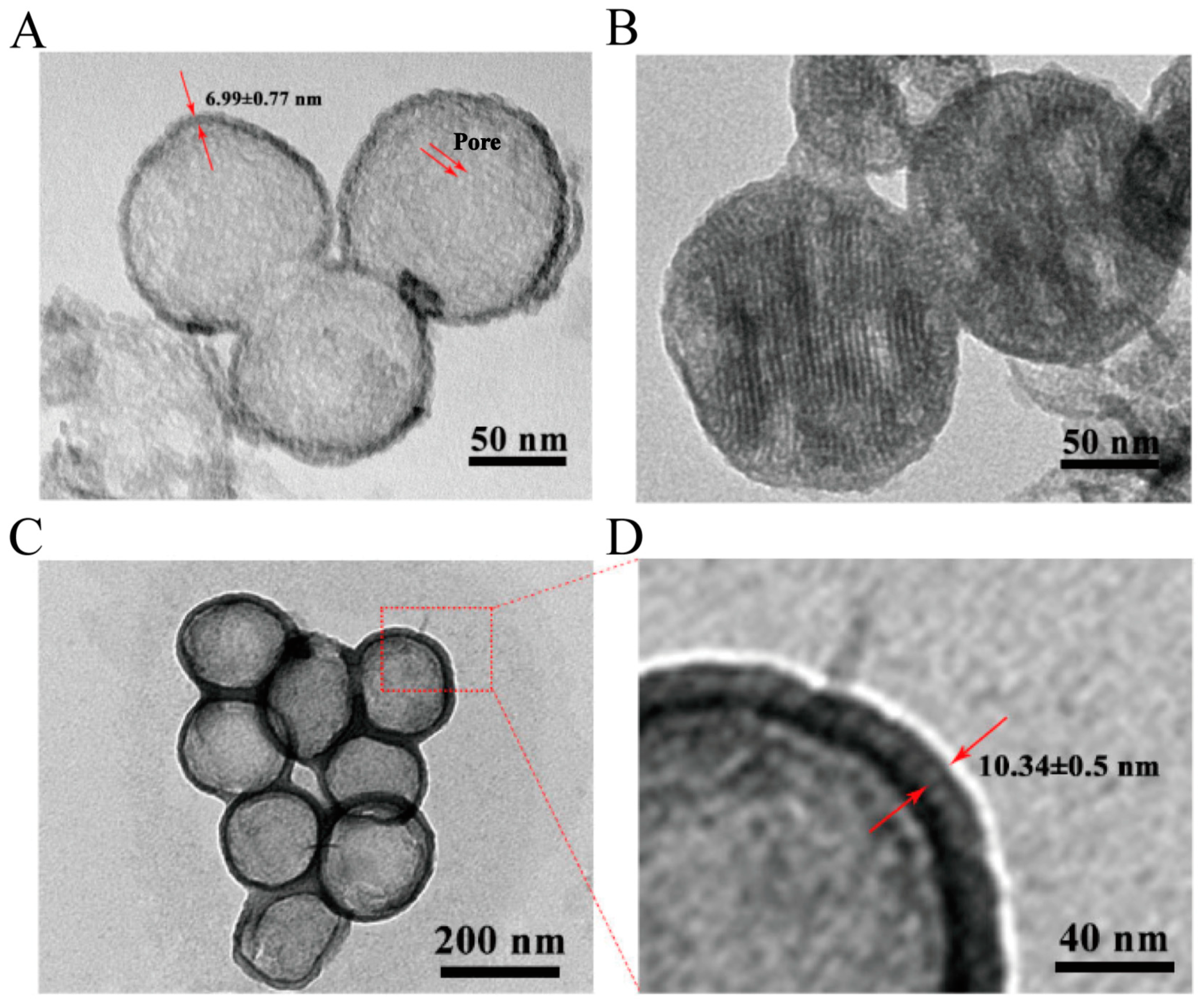
Pharmaceutics | Free Full-Text | Fabricating a PDA-Liposome Dual-Film Coated Hollow Mesoporous Silica Nanoplatform for Chemo-Photothermal Synergistic Antitumor Therapy

Activities of the USP Microbiology and Sterility Assurance Expert Committee During the 2005–2010 Revision Cycle | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Sterility Assurance and Sterility Assurance Level | PDA Journal of Pharmaceutical Science and Technology

Future of Consensus Standards in Biotechnology | PDA Journal of Pharmaceutical Science and Technology

About the PDA Journal of Pharmaceutical Science and Technology | PDA Journal of Pharmaceutical Science and Technology

Viral contamination in biologic manufacture and implications for emerging therapies | Nature Biotechnology

PDF) Demonstrating PQS Effectiveness and Driving Continual Improvement: Evidence-Based Risk Reduction
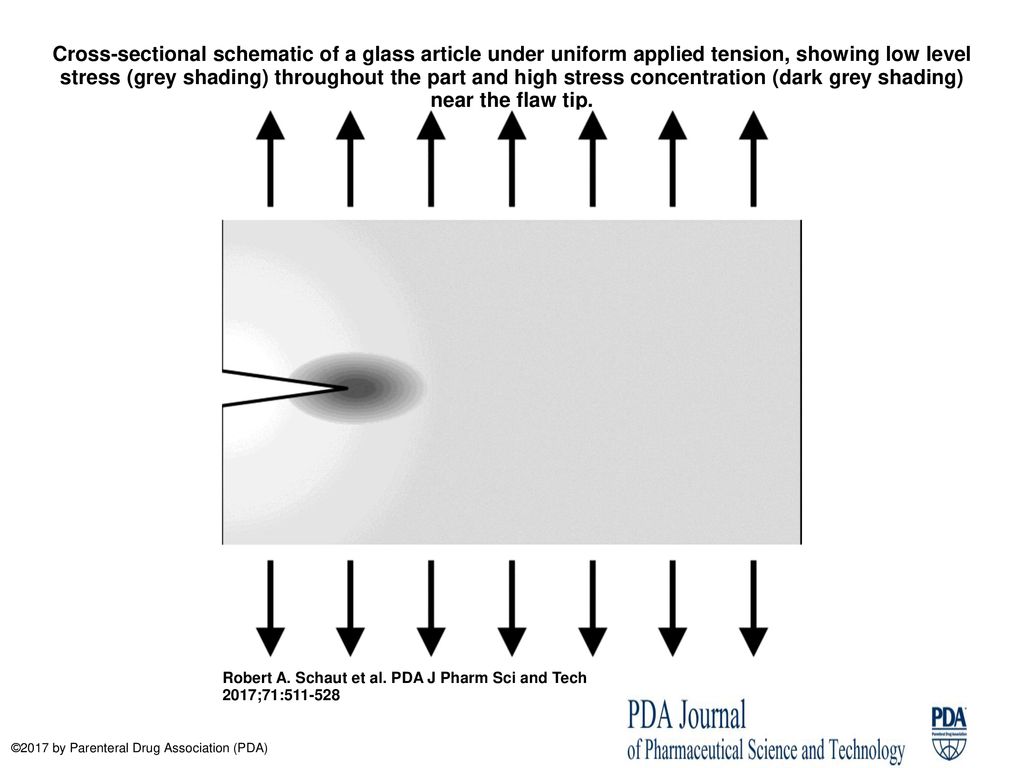
Cross-sectional schematic of a glass article under uniform applied tension, showing low level stress (grey shading) throughout the part and high stress. - ppt download