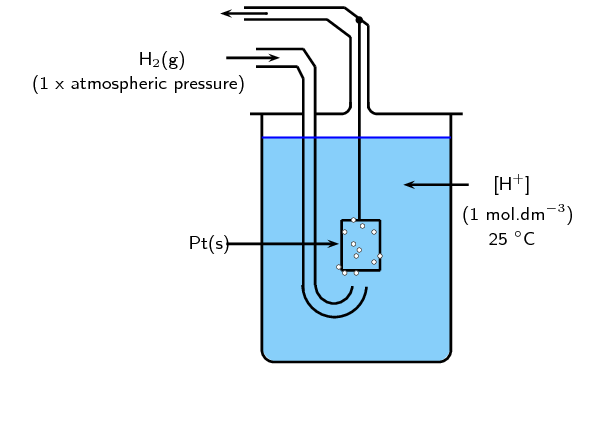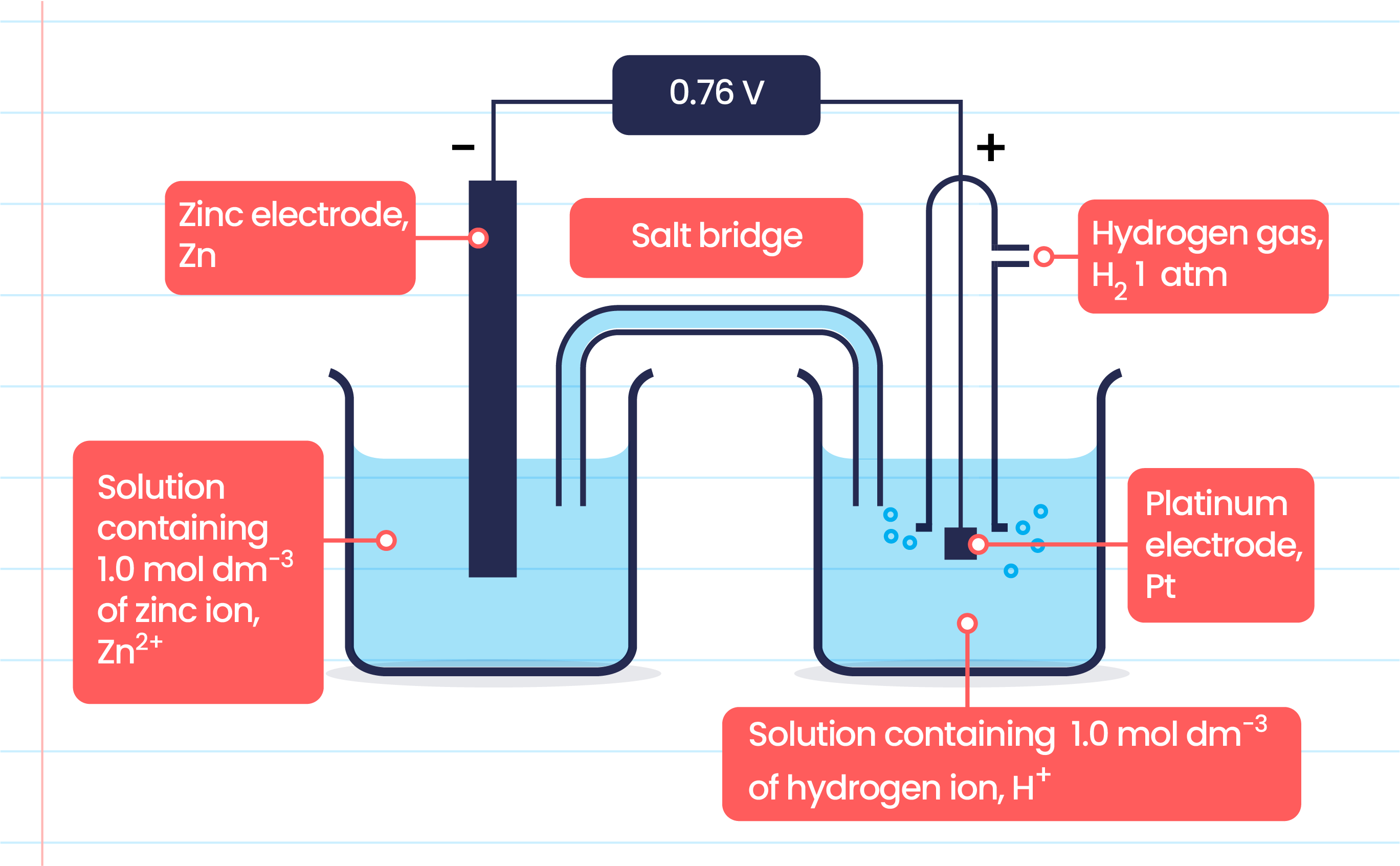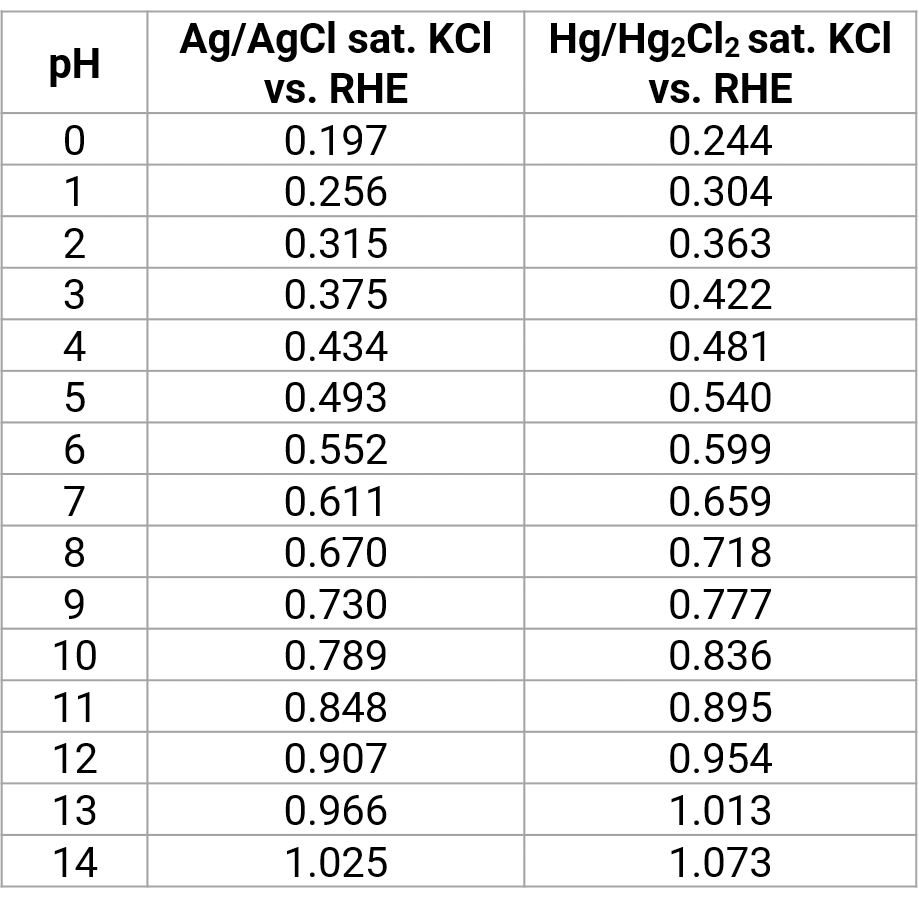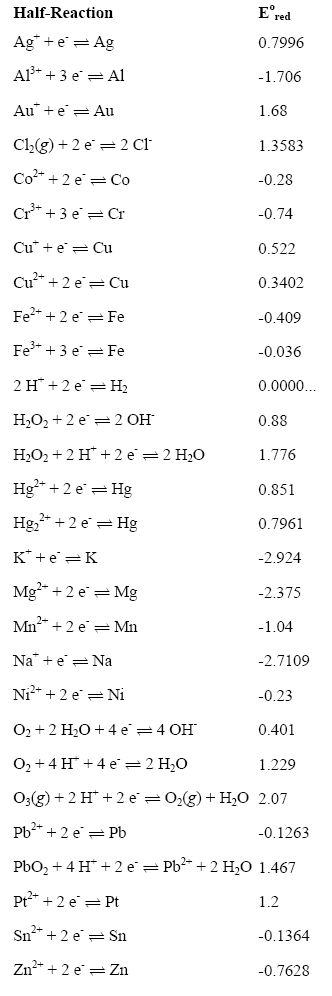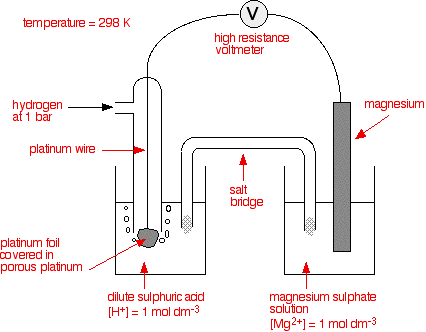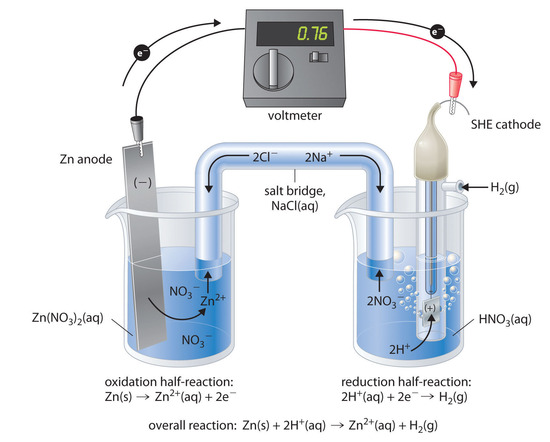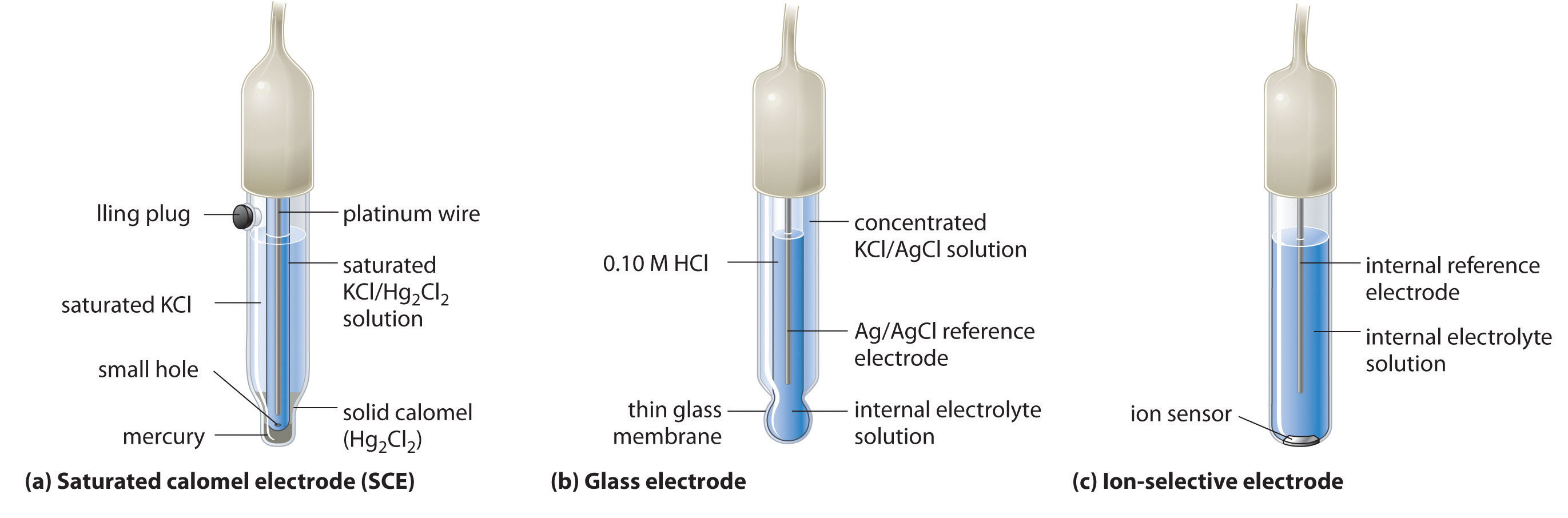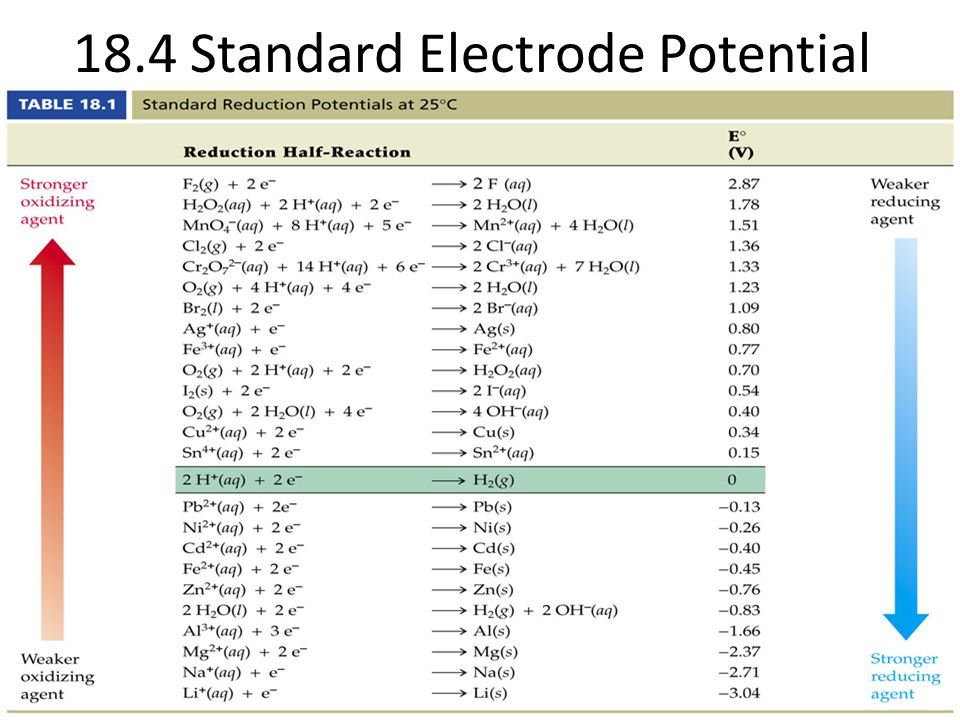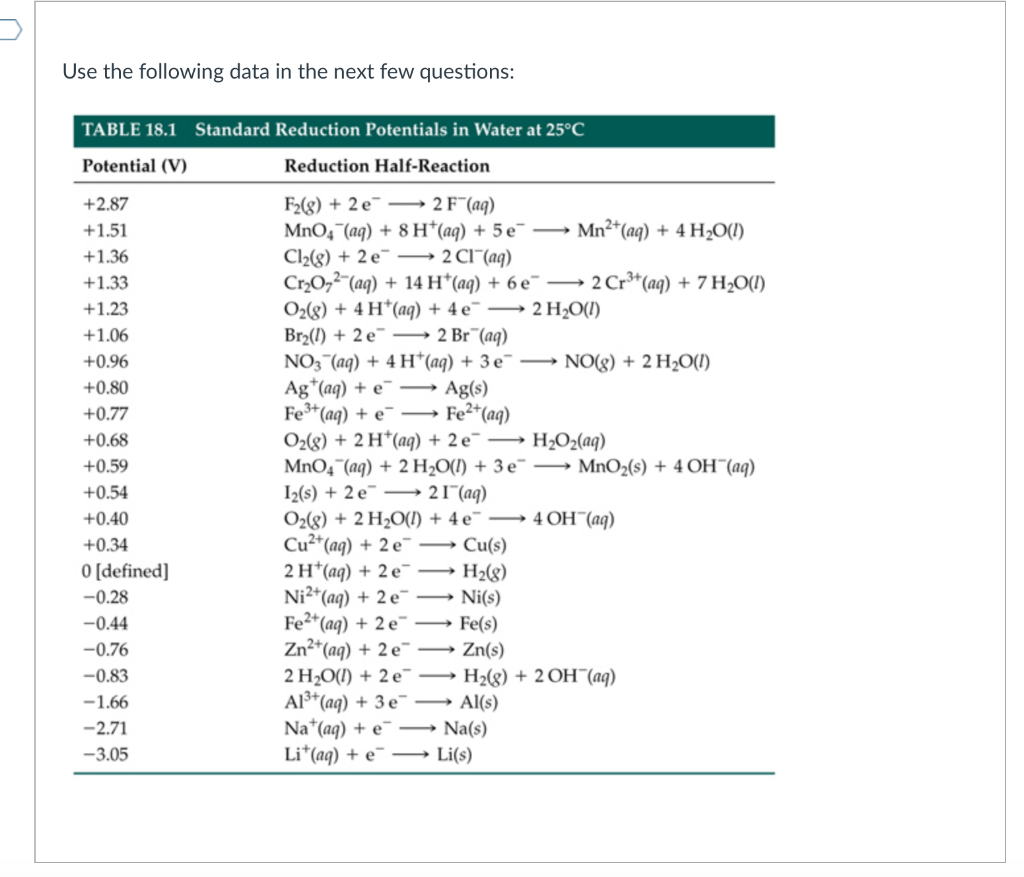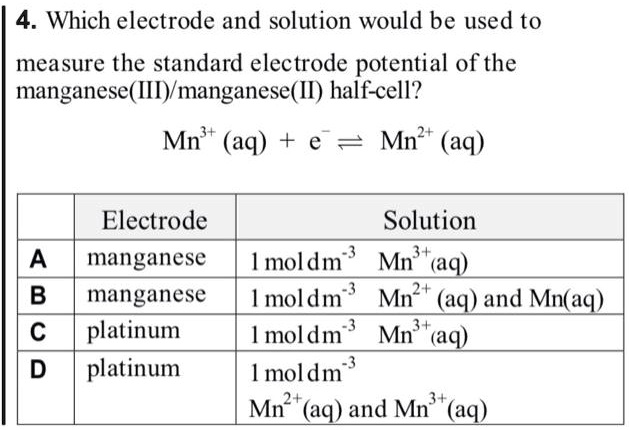
SOLVED: 4 Which electrode and solution would be used to measure the standard electrode potential of the manganese(III)manganese(II) half-cell? Mn' (aq) + = Mn?* (aq) Electrode manganese manganese platinum platinum Solution moldm
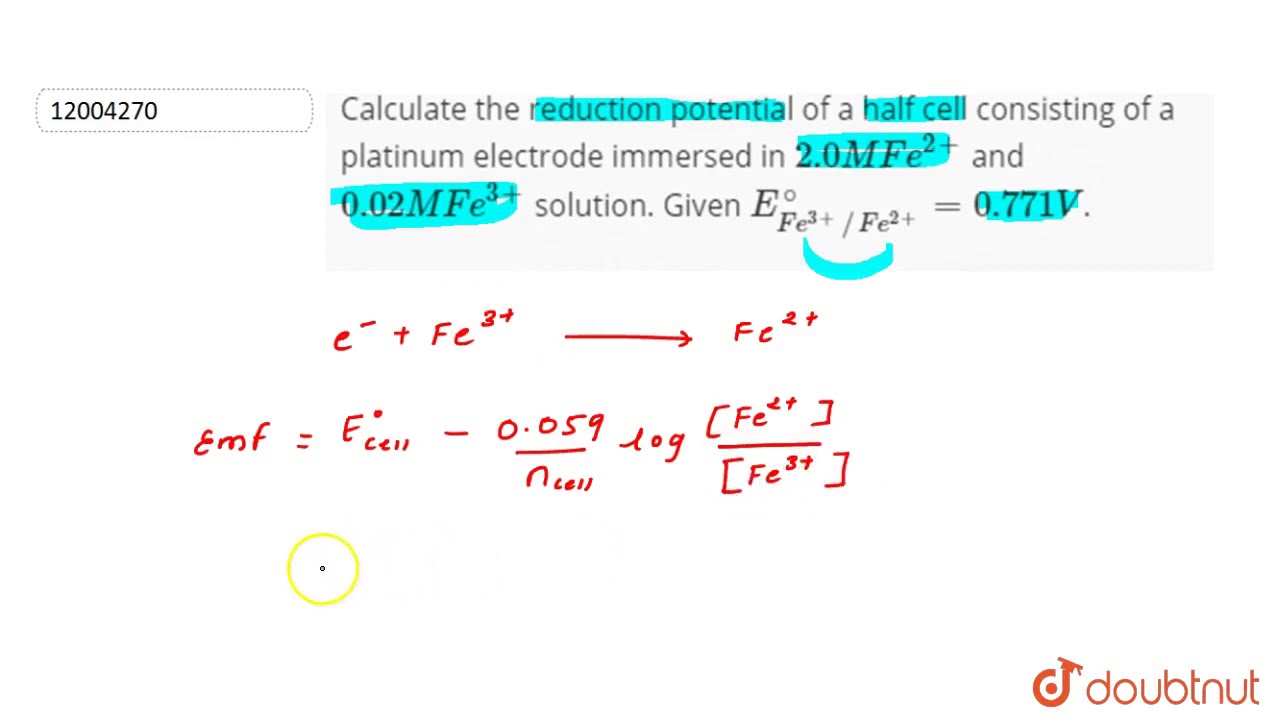
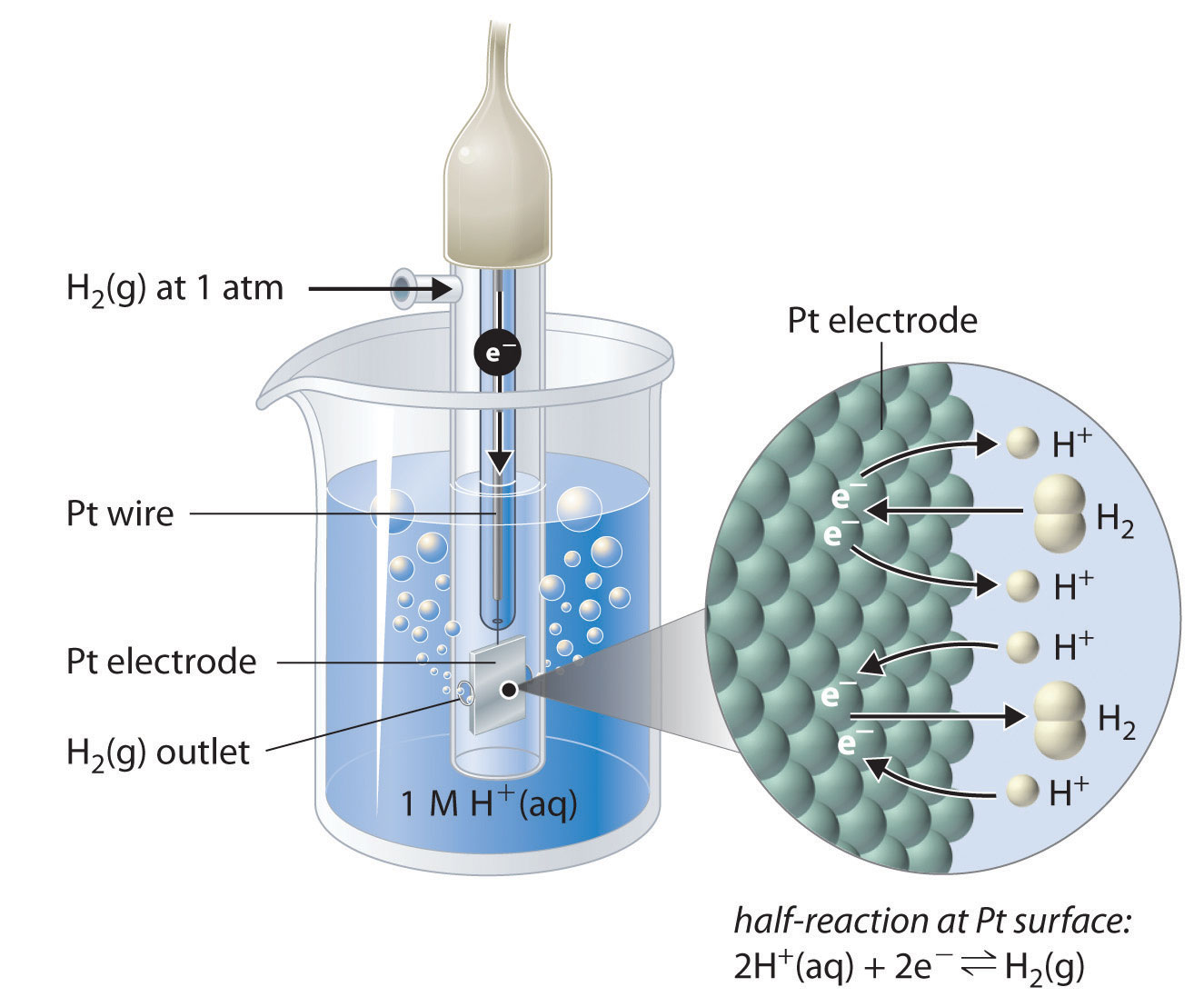
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in - YouTube

Stability of the potential of bare platinum electrode measured in 0.05... | Download Scientific Diagram

Applicability of Platinum as a Counter-Electrode Material in Electrocatalysis Research | ACS Catalysis
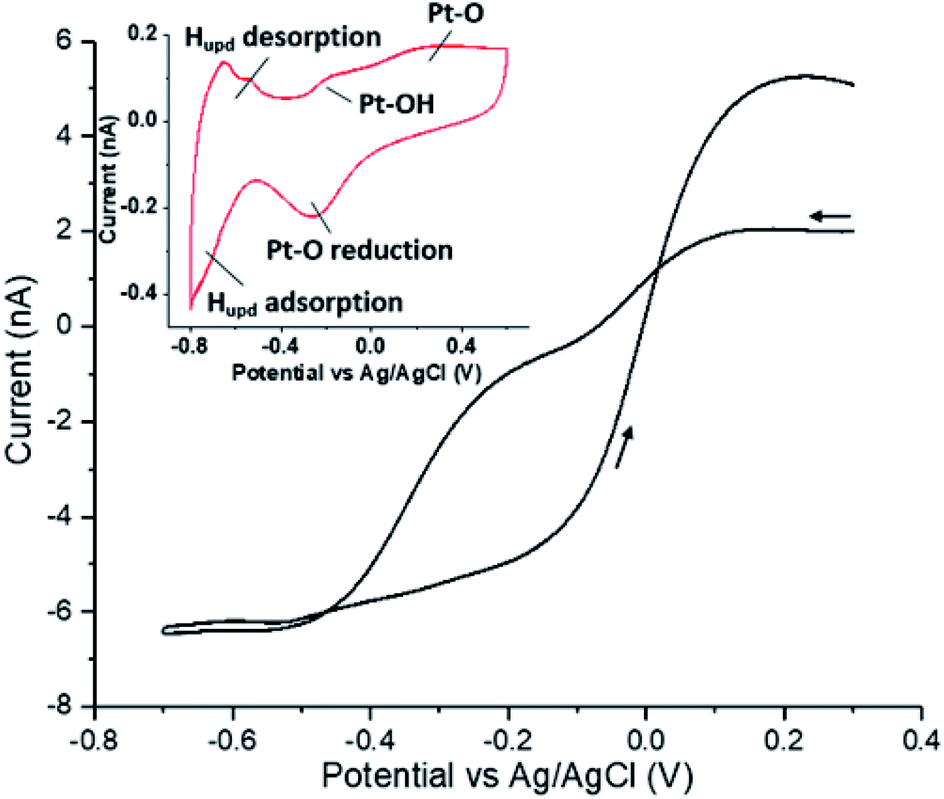
Hydrogen peroxide reduction on single platinum nanoparticles - Chemical Science (RSC Publishing) DOI:10.1039/D0SC00379D

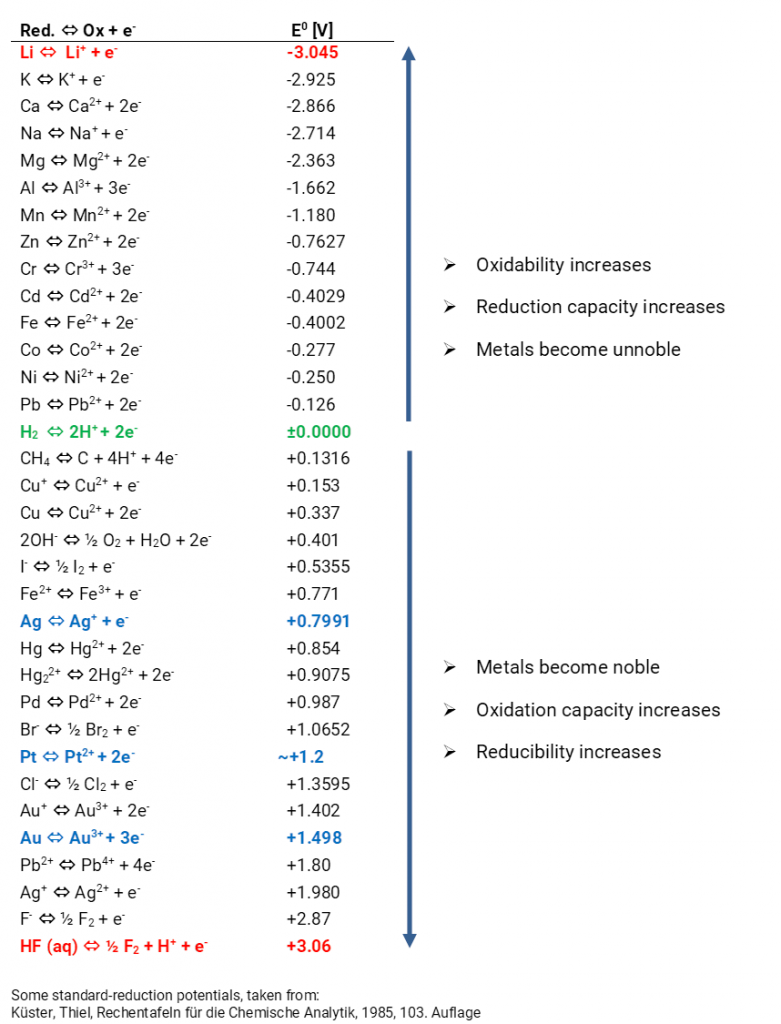
Chemistry - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different elements

Why is platinum black used in standard hydrogen electrode and how does it help to achieve equilibrium in the cell? - Quora