Noble GasesNoble Gases Helium- Not reactive. Neon- Not reactive. Argon- Not reactive. Krypton- Not reactive. Xenon- Not reactive. Radon- Not. - ppt download

Inert Gas Overview, Types & Examples | What are Noble Gases? - Video & Lesson Transcript | Study.com

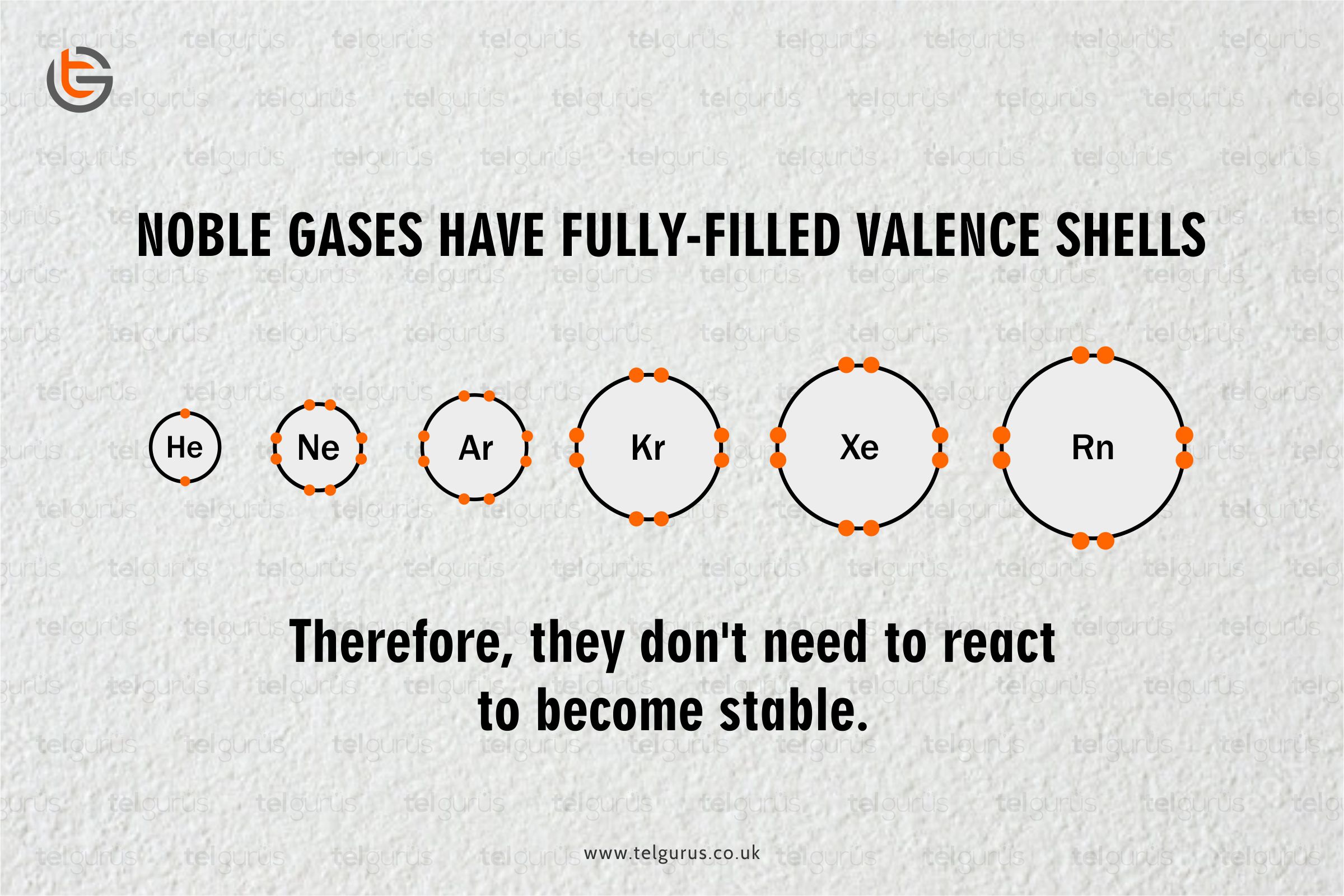
What is a property of the Noble Gases? * A. They are not reactive because their outer valence electron - Brainly.com

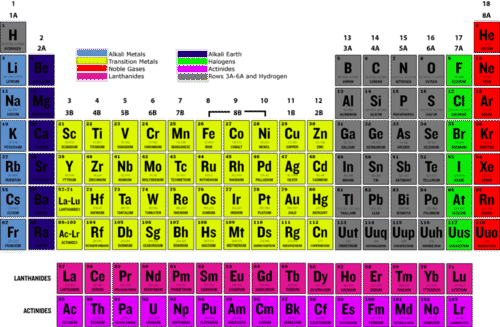
Group 0/18 NOBLE GASES physical properties uses helium neon argon krpton xenon radon melting points boiling points atomic radii density inertness explained gcse chemistry KS4 science igcse O level revision notes

Noble GasesNoble Gases Helium- Not reactive. Neon- Not reactive. Argon- Not reactive. Krypton- Not reactive. Xenon- Not reactive. Radon- Not. - ppt download

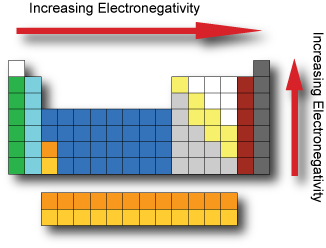
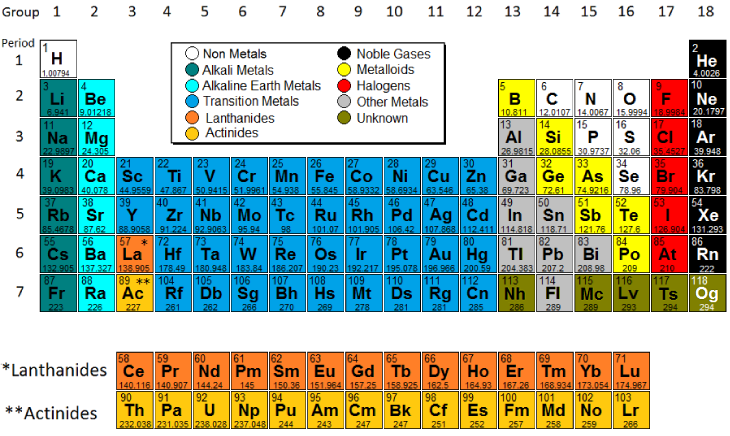
Periodic table extract, showing the non-metallic elements: the groups... | Download Scientific Diagram