
Metals like iron, copper, aluminium and zinc are given to a student. The correct decreasing order of reactivity of these metals written by the student is:

a). Arrange the following metals in order of their chemical reactivity, placing the most reactive - YouTube

gcse Reactivity series of metals, metallic activity order word/symbol equations of reactions of metals with air/oxygen, water, hydrochloric acid, sulphuric/sulfuric acid, nitric acid igcse KS4 science chemistry revision notes revising

Reactivity Differences of Rieke Zinc Arise Primarily from Salts in the Supernatant, Not in the Solids | Journal of the American Chemical Society

2.29 understand that metals can be arranged in a reactivity series based on the reactions of the metals and their compounds: potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, copper, silver and

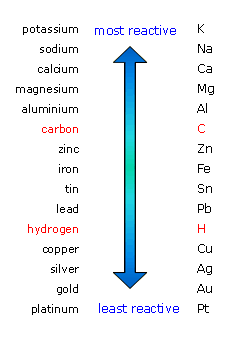





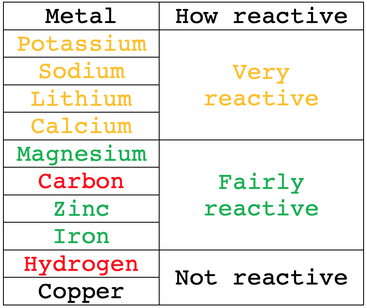
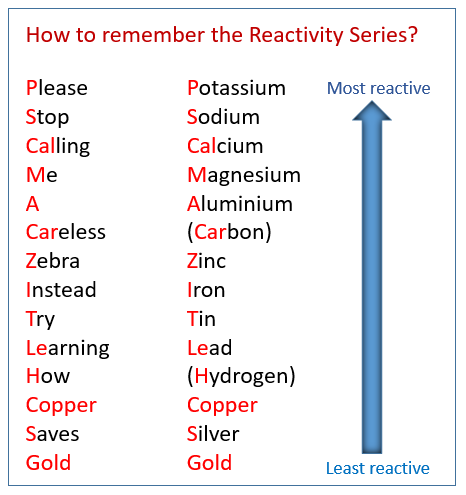
![Reactivity Series of Metals - Chart [and How to remember] - Teachoo Reactivity Series of Metals - Chart [and How to remember] - Teachoo](https://d1avenlh0i1xmr.cloudfront.net/b0a28f61-1fb3-456e-8ae8-e110e8999f99/reactivity-series-01.jpg)


